Australian Insulin Pump Choices
November 2022 update
Here’s the latest run-down. There are 6 pumps available new on the Australian market.
There are currently 6 pumps that can be purchased new through private health insurance and with reservoirs/infusion sets subsidised through NDSS. This list links short-cut directly to the relevant sections of this article.
- Accu-Chek Solo
- Tandem t:slim X2 (with Control-IQ closed-loop)
- YpsoPump (with CamAPS FX closed-loop)
- Medtronic 780G (with Medtronic closed-loop)
- DANA-i
- Omnipod DASH
There are also some older pumps in use, such as:
As usual I’ll run through them all with brief descriptions. Then I’ll mention other pumps we’re aware of which are not available at this point.
EOFlow, Insight, Kaleido, Mobi (t:sport), t:slim X3, iLet, Terumo, DIA:CONN, Sigi, Equil.
A note about “closed-loop” systems referred to below: those are systems which use your CGM data to dynamically adjust your pump’s delivery of insulin. These are not the mythical complete “artificial pancreas” replacement, but they do add a lot of automation to help you manage your diabetes and sometimes they feel like they get pretty close. For information about the open protocol closed-loop systems, see my separate page on the Australian options.
The hurdles for pumps to be sold in Australia
For new pumps to be available in Australia, the first hurdle they need to pass is registration with the Therapeutic Goods Administration (TGA), after which suppliers are legally allowed to talk about them.
The next hurdle is generally getting listing on the Federal Department of Health’s Prostheses List so they’re eligible for supply by health insurance. As of the time of writing, the Prostheses List’s latest update is effective from the 1st of November 2022.
Subsidised pumps available new in Australia
The following pumps are available new. Usually they’re bought through private health insurance, where they’re classified as prosthetic devices. Not all private health policies cover 100% of the cost: check with your own insurer as to coverage. But if they cover “insulin pumps” then they’ll cover all of these.
Accu-Chek Solo
 Unfortunately the Solo has not been available for a while due to supply issues (although current users are still supported). Hopefully this gets resolved soon.
Unfortunately the Solo has not been available for a while due to supply issues (although current users are still supported). Hopefully this gets resolved soon.
This tubeless patch pump has multiple components, starting with a cannula patch onto which the pump clips. The cannula is 90˚ teflon with two length options. There’s a disposable 200U reservoir (which includes the battery for the pump body).
The pump body nominally lasts 4 months. The Aviva Solo handset has an integrated BG meter (although you can enter corrections using a BG value you’ve retrieved from another meter or CGM). Because the pump body lasts for 4 months, when you buy the pump each year you will receive three bodies. Plus if it’s the first year you’ll receive a handset.
The Solo has buttons each side, which can be pressed together in patterns to administer simple boluses even without the handset.
Insulin delivery
Hourly basal rates: 0.1-5.0 U/hr in 0.01 increments, 0.1 U/hr increments above that.
Boluses: 0.2 U minimum, 0.05 U increment.
Bolus speed: varies between 1-2.5 U/min.
Consumables
The 9mm and 6mm teflon cannulas, and 200U reservoirs (which contain the battery that powers the pump) are available through NDSS. A dedicated inserter device for the infusion sets is supplied with the pump.
Waterproof?
 The Solo pump is not waterproof: you are expected to remove it (leaving the cannula patch in place) for swimming and showers. This is primarily because the tiny battery has to use a “zinc-air” chemistry to get enough capacity, and without access to air the battery stops working. There’s a small vent on the outside of the pump, that if you covered with tape would kill the pump.
The Solo pump is not waterproof: you are expected to remove it (leaving the cannula patch in place) for swimming and showers. This is primarily because the tiny battery has to use a “zinc-air” chemistry to get enough capacity, and without access to air the battery stops working. There’s a small vent on the outside of the pump, that if you covered with tape would kill the pump.
The pump’s ingress-protection rating is only IP22!
Closed loop?
Support for the Solo is on the cards for the Diabeloop commercial closed-loop system in Europe, but that is not accessible in Australia.
More info
Official info: Accu-Chek
Peer support (via FB): Accuchek Solo Pumpers Australia
Tandem t:slim X2
 This pump is a small pump with a touchscreen interface. The aluminium case is very robust, and gives it a slight heft in your hand. The internal battery is recharged by USB (typically topped up at least every few days).
This pump is a small pump with a touchscreen interface. The aluminium case is very robust, and gives it a slight heft in your hand. The internal battery is recharged by USB (typically topped up at least every few days).
Note that Apidra insulin is not compatible with the Tandem reservoirs (it’s not officially listed for pump use in Australia, but is sometimes used in other pumps). Apidra fails catastrophically in this pump.
Fiasp is officially not supported in the t:slim either, although some people apparently do use that successfully.
Insulin delivery
Basal rates in up to 16 arbitrary blocks (don’t have to be hourly): starting at 0.100 U/hr, with 0.001 increments above that.
Bolus speed: varies between 1.43-2.97 U/min.
However, the minimum basal rate is 0.100 U/hr (or 0), and this can cause issues for some paediatric use as this minimum also applies to temporary basal rates. Note that even if your basal rate is 0.6 U/hr, that minimum 0.1 still applies to any temp basal. Thus the lowest TBR you’d be able to apply would be 17%. Not 10%. If your basal rate was 0.3 U/hr you’d only be able to drop to 34%. The t:slim X2 is currently the only pump which limits temp basals like this.
Consumables
The Tandem reservoirs are compatible only with the Tandem t:lock infusion sets. The older reservoirs that could be used with any luer-lock infusion set have been discontinued. The Tandem infusion sets are:
- VariSoft 30 (teflon, angled: same as Medtronic Silhouette, Accu-Chek TenderLink)
- AutoSoft 30 (teflon, angled)
- AutoSoft 90 (teflon: same as Medtronic Mio, YpsoPump Inset, DANA Inset II)
- TruSteel (steel: same as Medtronic Sure-T)
These are all available through NDSS.
Waterproof?
The t:slim X2 is rated as IP67 waterproof, which is not quite as robust as the IPX8 rating of most other current pumps. See this article for a discussion of waterproof ratings. Mind you, the IP67 rating does still allow for short-term immersion in water up to a metre deep.
Note that the rubber door that covers the USB port is not actually part of the waterproofing: don’t panic if it doesn’t close or breaks off.
Tandem updates
A touted feature of this pump is the ability to update its firmware at home via USB without having to have the whole pump replaced/serviced, and this has already been used to add features to the pump at no direct cost during its 4-year warranty period. Yes, “free” upgrades (although there may be costs involved in getting doctors to sign off on it).
Apart from any minor updates, there are three major revisions we’re aware of:
1. Dexcom G5
The initial version of the pump acted as a Dexcom G5 CGM receiver. This seems to be the version that resulted in the December 2020-April 2021 suspension of its TGA registration. This version is no longer available new. And once you update your pump to a later version, you cannot go back. In any case the Dexcom G5 CGM was discontinued in March 2022.
2. Basal-IQ
This version was introduced in 2020, changed the CGM to use the Dexcom G6, and won’t connect to G5. It uses this to not just auto-populate the bolus advisor as with G5, but also to implement low-glucose suspend.
It was released in the US in August 2018, so there’s a lot of user experience with it online even before it reached Australia in 2020.
Basal-IQ suspends basal deliveries if your BG is below 3.9 mmol/L, or predicted to go below 4.4 mmol/L within 30 minutes, and should resume when it knows you’re coming back up. If it’s been suspended for 2 hours then it will resume for 30 minutes. Its predictions of where your BG is going are based only on the CGM data (not including carb or insulin information).
Do note that if it suspends basal deliveries it also cancels any current extended bolus. Temporary basal rates can be used instead (they resume) although you need to manually disable them when you no longer need them (extended boluses stop after a specified time).
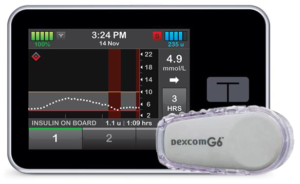
3. Control-IQ
Control-IQ implements a closed-loop system where the pump not only decreases insulin supply (not the brute-force suspend used by Basal-IQ) if you’re heading low, but also increases it if you’re running high. It still needs you to manage it, but it does a lot of the moment-to-moment decision making for us.
In the US it can also link to the t:connect phone app via Bluetooth, allowing viewing and uploading of pump data without getting the pump out of your pocket. It would be nice if that became available in Australia at some point.
Control-IQ reached Australia in 2021, but it was released in the US at the end of 2019, so again there’s a lot of user experience with it online.
User choice
t:slim X2 pumps can currently be ordered with Basal-IQ or Control-IQ.
External closed loop?
The t:slim X2 pump is not compatible with any external closed-loop system. Obviously Control-IQ is its own closed-loop system.
More info
Official info: AMSL Diabetes
Simulator app (for both iOS and Android): in app stores (linked from AMSL).
Peer support (via FB): Tandem TSlim X2 Australia+New Zealand
YpsoPump
 The YpsoPump (from Ypsomed) is a tiny unit which links to an app app on your phone via Bluetooth. For a long time the pump and mylife app have had a one-way connection: it needed the user to use the pump’s touchscreen to issue boluses, but does most of the the rest of the management and connection from the phone (including carb/bolus calculations). But now there’s a change happening.
The YpsoPump (from Ypsomed) is a tiny unit which links to an app app on your phone via Bluetooth. For a long time the pump and mylife app have had a one-way connection: it needed the user to use the pump’s touchscreen to issue boluses, but does most of the the rest of the management and connection from the phone (including carb/bolus calculations). But now there’s a change happening.
The Ypsomed-provided options are:
- Their mylife app. Supports the old YpsoPumps (which were supplied from 2018-2022) and the new “Dose” versions that can be driven from your phone. Unfortunately the TGA has not yet approved this remote-bolus functionality yet, so there’s little difference at this point. But…
- mylife CamAPS FX. A commercial closed-loop system that supports the new “Dose” pumps. This is available now and works.
In-warranty YpsoPump users are able to get their pumps upgraded to the Dose version at no cost. Like most such upgrades, this does not extend the warranty.
CGM integration
The Mylife app runs on pretty much any iPhone or Android phone. Since November 2021, if your phone is on a shorter list of compatible Android devices (similar to Dexcom’s list of supported Android devices) the Mylife app can be used to control the Dexcom G6 CGM (it replaces Dexcom’s software). The name for this upgrade has been “Mylife Assist”, but it’s implemented within the regular “Mylife” app.
This function is expected on iPhones at some point, but currently it’s Android only.
Having the CGM data integrated into Mylife means that the BG data is available for bolus calculations, and is also uploaded to the Mylife Cloud along with all the pump data. You have the choice to give your clinic access to view your cloud data, at which point you never have to remember to upload your pump before an endocrinologist/DE meeting: the data is incrementally uploaded by the app every day.
Mylife Dose
I’ve been hoping to see the Mylife Dose upgrade in 2022, but it’s still a mystery as to when the TGA’s going to sign off on it. It will allow us to bolus directly from the app without needing to lay a hand on the pump.
CamAPS FX
The recently-updated YpsoPump is supported by CamAPS FX, which is a closed-loop application that currently runs on an Android phone and uses the Dexcom G6 CGM. The rumour mill suggests that an iPhone version of CamAPS might be available in 2023. But for now it’s Android only. Many iPhone users have been getting small/cheap Android phones for this (just like many users of AndroidAPS).
CamAPS has been used with DANA pumps in Europe for some time, but with that there’s a monthly subscription cost. With the YpsoPump there is no added cost. This software can be found in the Google Play Store and installed on a phone for free. It can be configured to talk to either an actual G6 transmitter or to a CGM simulator, and to either an actual YpsoPump Dose or a simulator (for training purposes).
CamAPS is the only closed-loop system that’s TGA-approved for use from ages 1+, and in pregnancy. It’s been getting a lot of positive feedback.
Insulin delivery
Hourly basal rates: 0.02-1.0 U/hr in 0.01 increments. 1.0-2.0 U/hr (0.02 increments), 2.0-5.0 (+0.05), 5-15 U/hr (+0.1), and 0.5 U/hr increments above that.
Boluses: 0.1 U minimum, 0.1 U increment.
Bolus speed: 33.3 U/min.
Consumables
The reservoir holds up to 160U of insulin, and the basal rates can be set to 0.01 U/hr increments in 1-hour blocks. The pump uses three types of infusion set:
- Orbit soft (teflon)
- Orbit micro (steel)
- YpsoPump Inset (same as Medtronic Mio, Tandem AutoSoft 90, DANA Inset II, and Animas Inset II)
All these consumables are available through NDSS. A 2018 article on this site talks about the Orbit infusion sets.
The reservoir is smaller than in some other pumps, although 160U is enough for many people’s requirements. But the reservoirs are quick and easy to swap in (without having to replace the infusion set at the same time). So I think the 160U limit is unlikely to be a problem for most people. Plus the reservoirs are glass (unlike the plastic of other pumps) and as such are approved for longer-term insulin storage. The tested and approved use includes the ability to pre-fill reservoirs and keep them in your fridge for up to 4 weeks.
Of the people I have spoken to who have expressed reservations about the 160U reservoirs, it turns out most of them have an idea of “a reservoir change” based on their experiences with other pumps. Procedures where you need to draw insulin out of a vial and into the reservoir, plus often change the infusion set at the same time. The Ypsomed changes are much simpler than that! When using this pump I routinely swap to a new reservoir and be back running in under 2 minutes.
Batteries
The YpsoPump uses an alkaline AAA battery. Lithiums are rejected by the pump (they have a different voltage), but the alkaline cells last about a month and are even supplied by Ypsomed so you don’t need to go to the supermarket, so that doesn’t seem inconvenient to me.
Waterproof
The YpsoPump is rated at IPX8. The manual describes “immersion to a depth of 1m for up to 60 minutes”, and says that “for water sports that cannot comply with these specifications (e.g. diving)” we should disconnect.
More info
Official info: Ypsomed Australia
Simulator app: YpsoPump Explorer (for both iOS and Android).
Peer support (via FB): YpsoPumpers Australia
Medtronic 770G
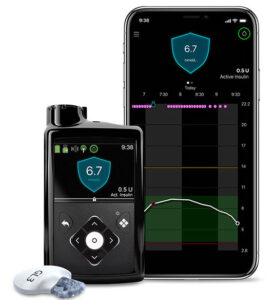 The Medtronic 770G is a development from the earlier 670G. It runs a closed-loop algorithm using the Medtronic CGM to try to control both highs and lows.
The Medtronic 770G is a development from the earlier 670G. It runs a closed-loop algorithm using the Medtronic CGM to try to control both highs and lows.
The looping algorithm is the same as the 670G, with the same fixed 6.7 mmol/L target. But it can be software-upgraded to the enhanced algorithms of the 780G pump (see below).
The 770G does have a few extra features over the 670G, such as:
- It has Bluetooth integrated into the pump. This is used to link the pump to your mobile phone (with the CareLink app uploading to Medtronic’s cloud servers). No longer do we have to choose to either have the CGM connected to the phone or to the pump. We finally get to see it on both!
- The CGM uses the same Guardian3 sensors, but a new transmitter which uses Bluetooth (like the older Guardian Connect transmitters, but not apparently not identical).
- The CGM is managed by the pump though, which then reports the results to the phone. This is different to the way the Dexcom CGMs can talk to pump and phone independently. Unfortunately Medtronic does not support displaying the information from the phone app to smartwatches.
- The pumps are shipped with Accu-Chek Guide Link BG meters (instead of the older Contour Next Link 2.4) which connect to the pump via Bluetooth. The plain Guide meters (not the Link version) will link to the MySugr app on phones.
Insulin delivery
30-minute basal blocks, in 0.025 increments.
Boluses: 0.025 U minimum, 0.025 U increment.
Bolus speed: 1.5 U/min (“Standard”) or 15 U/min (“Quick”).
Consumables
The 770G can use either 300U or 180U reservoirs. Although the manuals only mention the 3ml reservoirs, the 1.8ml ones are still available through NDSS (and are required for some of the older Paradigm pumps).
The infusion sets are common across all the Medtronic pumps in this list and are available via NDSS. The available sets are:
- Mio (teflon: same as Tandem AutoSoft 90, YpsoPump Inset, DANA Inset II)
- Sure-T (steel: same as Tandem TruSteel)
- Silhouette (teflon, angled: same as Tandem VariSoft 30, Accu-Chek TenderLink)
- Quick-set (teflon)
Batteries
The 770G uses an AA battery. Either alkaline, lithium, or at a stretch NiMh rechargeable are supported. The pump does not ask for the battery type.
Note that carbon-zinc batteries (often labelled as “heavy duty”) are not supported.
Waterproof?
The 770G is described as coming from the factory as waterproof, able to be underwater to a depth of 3.6m for up to 24 hours. This is still IPX8. It’s the same rating as the older 640G and 670G pumps, and in fact the design of the pump body is unchanged.
However the manual does note that the waterproofing can be compromised by bumps/scratches and especially cracks in the pump. Many people over the years have reported their Medtronic pumps drowning due to cracks they hadn’t noticed before they got wet. So user beware!
Closed loop?
The 770G cannot be used with any external closed-loop system, but it has its own hybrid closed-loop system built-in.
More info
Official info: Medtronic
Peer support (via Facebook): Medtronic Support Group – Smartguard and related pumps
Medtronic 780G
This is Medtronic’s latest hybrid closed-loop pump. It’s the same hardware as the 770G (see above).
The internals of the algorithm has changed from the 670G/770G, and the BG target is adjustable between 5.6-6.7 mmol/L.
More info
Official info: Medtronic
Peer support (via Facebook): Medtronic Support Group – Smartguard and related pumps
DANA-i
 This is the current pump from SOOIL.
This is the current pump from SOOIL.
Consumables
The pump uses the same 300U reservoirs as the earlier DANA RS and R (and IIS) pumps that are outlined below and these are available through NDSS. Once it’s filled, a “linking screw” needs to be adjusted to the right level so the socket that rotates in the base of the reservoir enclosure can drive the screw and push the piston forward. This adjustment/calibration for each new reservoir is usually done using an “AutoSetter” device supplied with the pump.
For a long time the DANA pumps were mainly used with the Soft Release O infusion sets. These were made by Ypsomed and equivalent to the Orbit Soft set. The Orbit micro steel cannulae (available on NDSS without tubing) can be used with the DANA tubing if you needed to use those. However the Soft Release O has been discontinued. However if you still have any Soft O Release tubing, this can be used with all the Orbit cannula versions available from Ypsomed (through NDSS).
The infusion sets available through NDSS were overhauled in 2021:
- The “DANA Inset II“, which has a DANA connector but is otherwise identical to the Medtronic Mio, Tandem AutoSoft 90, Animas Inset II, and YpsoPump Inset.
- The “Easy Release“, which is a super-fine steel cannula available in 4.5mm and 7mm lengths). 4.5mm is even shorter than the Orbit micro’s 5.5mm (other models only go down to 6mm) and apparently infusion is very effective (as long as you don’t use large insulin volumes).
The pump uses a standard AAA alkaline battery. Compared with the earlier DANA pumps it is a bit bigger (“chunkier”) though. The pump does also have a few technical advances over the RS other than just a new battery, such as a new Bluetooth module with increased range.
Closed loop?
In Europe this pump has been supported by the commercial CamAPS FX looping system and by Diabeloop. But there’s no sign of these systems for Australia yet. It is supported by AndroidAPS. Do note that it does seem to be picky about Bluetooth 5.0 support in phones.
More info
Official info: Managing Diabetes
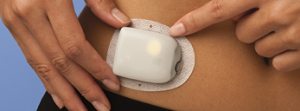
Insulet OmniPod DASH
 This tubeless patch pump was launched in Australia in August 2021. It has been used in Europe and the US for years, so there are plenty of user-experience stories online.
This tubeless patch pump was launched in Australia in August 2021. It has been used in Europe and the US for years, so there are plenty of user-experience stories online.
Because it is the pump, reservoir, and infusion site all combined into one disposable unit, there are a few differences in operation from traditional pump.
The internal reservoir is filled with a syringe prior to insertion, and it auto-inserts a teflon cannula after application. There are no cannula options: it’s a very short 6.5mm cannula at an angle. But the pump can be worn during swimming/showering/etc. If the pump detaches, a replacement pump is required. In fact every 3 days a whole new pump is required: the only permanent piece is the PDM (“Personal Diabetes Manager”) handset. Every month a new box of 10 pumps.
This model hasn’t fitted the traditional pump funding model in Australia, where there’s a “capital” component of the pump itself (and you buy 4 years of warranty support with it) and a “consumables” component. However NDSS subsidy of the Omnipods is apparently starting from December 1 2022.
 There have been two major versions of the Omnipod: “Eros” and “DASH”. The difference between these is the computer chip and the radio used to talk to the handheld PDM (and thus what they’re compatible with). The earlier Eros pods were never sold in Australia, although some people self-imported them from overseas. The major difference in them seems to be that the DASH pods use Bluetooth communications, whereas the old Eros pods used a custom 433 MHz radio link.
There have been two major versions of the Omnipod: “Eros” and “DASH”. The difference between these is the computer chip and the radio used to talk to the handheld PDM (and thus what they’re compatible with). The earlier Eros pods were never sold in Australia, although some people self-imported them from overseas. The major difference in them seems to be that the DASH pods use Bluetooth communications, whereas the old Eros pods used a custom 433 MHz radio link.
Usage
Unlike other pumps (including the Solo patch pump) there are no buttons on the pump itself. The only way to deliver insulin is using the PDM handset.
Errors occur at times with all pumps. With the OmniPod some can be resolved via the PDM, but some need to you remove the pump. In some error conditions the pump will alarm loudly, and the only recourse is to remove the pump and break the kill-switch panel (which is otherwise against your skin) with a nail/key/pin to silence it.
Insulin delivery
Each pod is filled with 85-200U of insulin, which has to last 72-80 hours (3 days plus 8 hours of “grace period”). As mentioned, the 6.5mm angled teflon cannula is auto-inserted when you activate the pod.
Up to 24 basal blocks (can be set to 30-minute boundaries) in 0.05 U/hr increments.
Boluses: 0.05 U minimum, 0.05 U increment.
Bolus speed: 1.5 U/min.
Waterproof?
The Omnipod is rated at IP28: down to a depth of 7.6 meters) for up to 60 minutes. You’re supposed to be able to shower, swim, etc without having to remove it.
Closed loop?
Officially the pods are only compatible with their PDMs. However…
The DASH works with AndroidAPS, and code for it is being tested in development versions of Loop and FreeAPS X. I wrote about some of my experiences with it in November 2021.
The Eros pods can be used with external closed-loop systems (Loop, FreeAPS X, and AndroidAPS) using a RileyLink/EmaLink/OrangeLink radio bridge (these replace the PDM). But Eros will never be supplied in Australia.
More info
Official info: Insulet
Peer support (via Facebook): Omnipod Users Australia
Other pumps in use in Australia
While not available new, the following pumps are still in use by many people.
Accu-Chek Combo
This pump is a relatively old design, but it has proven itself as a reliable performer over the years. The last of these was sold in September 2021, with warranty and support promised to continue for 4 years.
 The Combo system is comprised of a “Spirit Combo” pump along with a “Performa Combo” BG meter which is used as a remote control so the pump doesn’t have to be brought out of your pocket in normal use.
The Combo system is comprised of a “Spirit Combo” pump along with a “Performa Combo” BG meter which is used as a remote control so the pump doesn’t have to be brought out of your pocket in normal use.
Mind you, that Performa Combo does insist on a fresh fingerprick for each bolus decision. Although when used with AndroidAPS, the meter is replaced by a Bluetooth connection to your phone so the meter’s no longer required.
Insulin delivery
Hourly basal rates: 0.1-1.0 U/hr in 0.01 increments, 1.0-10 U/hr in 0.05 increments, 0.1 U/hr increments above that.
Boluses: 0.1 U minimum, 0.1 U increment.
Bolus speed: 12 U/min.
Consumables
The pump’s reservoir can hold up to 315U of insulin. The pump has a luer-lock connection for its infusion sets, so the currently-available (through NDSS) sets it can be used with are:
- Accu-Chek FlexLink (teflon)
- Accu-Chek Rapid-D (steel)
- Accu-Chek TenderLink (teflon, angled)
- Medtronic Silhouette (only 17mm version, but same as TenderLink)
- Medtronic Sure-T (steel)
- Medtronic Quick-set (teflon)
- Orbit soft (teflon), micro (steel)
- Cleo 90 (teflon)
Batteries
The Combo uses an AA battery. Either alkaline, lithium, or NiMh rechargeable are supported. After a new battery is inserted the pump asks for the battery type so it can calibrate the “low battery” indicator.
Waterproof?
The Spirit Combo has an IPX8 waterproof rating. Some people do take it swimming, although the manual’s official advice is quite conservative:
Avoid contact with water. Check daily that your Accu-Chek Spirit Combo insulin pump is not chipped, cracked or damaged in any way, and that the battery cover and the adapter are correctly closed. If the pump is chipped or cracked, water, dust, insulin, or other substances may enter your pump and lead to malfunction. Before any contact with water, disconnect and take off your pump.
Disconnect and remove your pump before taking a bath, or going into a whirlpool, shower, or swimming pool. Avoid exposing your pump to high humidity, such as in a sauna, as this could result in damage.
I spent years using the Combo. It was a very reliable pump for me. I always took the cautious line that I take the pump off before I head into water, but had faith that it would probably survive if I fall overboard when working around water.
Closed loop?
The Combo can be used with the AndroidAPS closed-loop system. This is not actively supported by Accu-Chek, but I suspect that from 2018-2021 most of the Combo sales in Australia were due to this compatibility.
More info
Official info: Accu-Chek
Peer support (via FB): AndroidAPS users, AussieLooping
Medtronic 670G
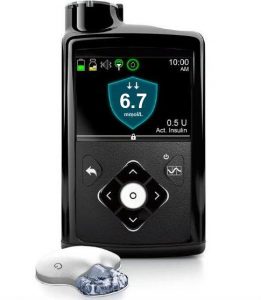 The 670G pump was Medtronic’s first closed-loop system. The pump acts as the receiver for the Guardian3 Link CGM, which it needs for the closed-loop functionality.
The 670G pump was Medtronic’s first closed-loop system. The pump acts as the receiver for the Guardian3 Link CGM, which it needs for the closed-loop functionality.
The pump is supplied with the same linked BG meter as the 640G. The meter, pump, and CGM sensors communicate via a radio protocol which is not Bluetooth.
The Guardian3 CGM has sensors that last for 7 days, and require calibration within every 12 hours.
Insulin delivery characteristics are the same as for the 770G.
Consumables
The reservoir, infusion sets, and battery are the same as the 770G/670G/etc.
Closed loop?
The 670G cannot be used with any external closed-loop system, but it has its own hybrid closed-loop system built-in.
With its CGM the 670G is able to have the same SmartGuard function as the 640G, as well as having “auto mode” which is their closed-loop system. Note that it is a first-generation looping system and lacks many of the configuration options and features of the open protocol loop systems (such as the inability to customise the target BG level) although it is approved by the regulators and available without you having to assemble it yourself.
More info
Peer support (via Facebook): Medtronic Support Group – Smartguard and related pumps
Medtronic 640G
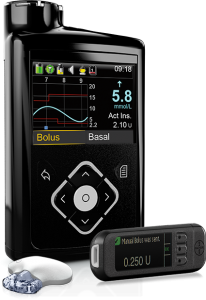 This pump originally integrated with Medtronic’s Guardian2 Link CGM, but also supports the Guardian3 Link that was introduced with the 670G.
This pump originally integrated with Medtronic’s Guardian2 Link CGM, but also supports the Guardian3 Link that was introduced with the 670G.
However, it’s mainly used these days without CGM. If a 640G does have CGM connected, it can use that data for “SmartGuard” which in this pump is their predictive-low-glucose-suspend (sometimes referred to as a “hypo minimiser”).
The pump is supplied with a “Contour Next Link 2.4” BG meter, which links wirelessly (not using Bluetooth) to the pump.
Consumables
The reservoir, infusion sets, and battery are the same as the 780G/770G/670G/etc.
Waterproof?
The 640G has the same waterproofing as the 780H/770G/670G/etc.
Closed loop?
The 640G is not compatible with any closed-loop system.
More info
Official info: Medtronic
Medtronic Paradigm
These were Medtronic’s earlier generation of pumps. The consumables (reservoirs and infusion sets) are still used with the current 770G.
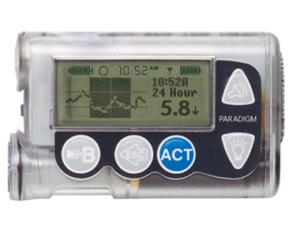 The “Veo” 554 and 754 pumps were the last versions of these, but the earlier 522, 515, 722, and 715 look very similar.
The “Veo” 554 and 754 pumps were the last versions of these, but the earlier 522, 515, 722, and 715 look very similar.
The reservoir in the 554 can hold up to 180U of insulin, and the 754 can hold up to 300U (or use the 180U option).
The Veo pumps support Medtronic’s old “Enlite” CGM system (uses the same sensors as the Guardian2 CGM, but a different “MiniLink” transmitter) and they have a primitive low-glucose-suspend function (which suspends when you get to the boundary point, not when the pump predicts you’re soon going to get there).
These pumps were originally rated with IP67 dust/waterproofing, but especially given their age I would not regard any of them as waterproof today.
Insulin delivery
30-minute basal blocks, in 0.025 increments.
Boluses: 0.025 U minimum, 0.025 U increment.
Bolus speed: 1.5 U/min. The “Veo” models halve that speed for boluses <1 U.
Consumables
The reservoir and infusion sets are the same as the Medtronic 770G/etc, and are available through NDSS. The 5xx pumps only support the smaller 1.8 mL reservoirs though.
Batteries
Officially the only batteries supported were alkaline AAAs. The on-screen battery-level indicator is calibrated for these. However many loopers are using lithium AAAs which last a lot longer (but if you rely on the LCD battery indicator drop from full to empty very quickly, which I think is why Medtronic originally said these were not supported). The looping software has its own recalibrated indicator. I personally used lithium AAAs in these pumps for a long time.
Closed loop?
The latest firmware versions of these pumps are not compatible with any external closed-loop systems. Older versions with firmware up to 2.7A are “loopable” and work with all four opensource closed-loop systems (OpenAPS, Loop, and AndroidAPS, FreeAPS X).
The 512/712 models are only compatible with OpenAPS and AndroidAPS (not Loop), but the Paradigm 515/522/554 and 715/722/754 models work with all four.
DANA R and RS
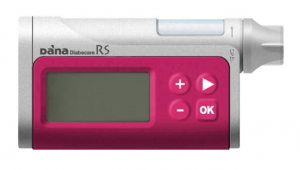 The DANA R and RS pumps look almost identical. The main differences are the R’s use of Bluetooth 2 rather than the lower-power Bluetooth LE of the RS, and a correspondingly-worse battery life (approximately a third of the lifespan). The pumps have a 300U insulin reservoir. The RS was superseded in Australia by the DANA-i in 2022, but they all use the same consumables (available through NDSS).
The DANA R and RS pumps look almost identical. The main differences are the R’s use of Bluetooth 2 rather than the lower-power Bluetooth LE of the RS, and a correspondingly-worse battery life (approximately a third of the lifespan). The pumps have a 300U insulin reservoir. The RS was superseded in Australia by the DANA-i in 2022, but they all use the same consumables (available through NDSS).
Insulin delivery
Hourly basal blocks: minimum 0.04 U/hr, with 0.01 increments.
Boluses: 0.05 U minimum, 0.05 U increment.
Bolus speeds. R: 5 U/min. RS: 4, 2, or 1 U/min.
Batteries
The special batteries for the pump are still supplied in each box of reservoirs.
Closed loop?
Both the R and RS pumps are compatible with the AndroidAPS closed-loop system.
Other insulin pumps
That’s it for the current Australian pump options, but there are some other pumps around the world which Australian folk keep asking about.
V-Go: a non-T1D pump
 The V-Go is available in Australia, but is designed for use in Type 2 diabetes, not Type 1. It is a simple mechanical (spring-driven) device for 1-per-day use, with a fixed infusion rate. I have previously written about the V-Go.
The V-Go is available in Australia, but is designed for use in Type 2 diabetes, not Type 1. It is a simple mechanical (spring-driven) device for 1-per-day use, with a fixed infusion rate. I have previously written about the V-Go.
EOFlow
 This patch pump from Korea got some press in March 2019 when it got “Breakthrough Device” designation from the US FDA. It is apparently available in South Korea now, but it’s a long way from other markets.
This patch pump from Korea got some press in March 2019 when it got “Breakthrough Device” designation from the US FDA. It is apparently available in South Korea now, but it’s a long way from other markets.
Apparently research and testing is continuing with a version that integrates a CGM sensor as well as infusion cannula into a single unit for closed-loop operations.
Accu-Chek Insight
 This pump is used in Europe, although supply has ceased in the UK at least. Like the other Accu-Chek pumps it has a remote handset/meter which links to it via Bluetooth.
This pump is used in Europe, although supply has ceased in the UK at least. Like the other Accu-Chek pumps it has a remote handset/meter which links to it via Bluetooth.
The pump needs its own collection of Accu-Chek infusion sets (the end of the pump is actually part of the infusion set tubing).
It uses the same cartridges as the YpsoPump, but the pre-filled NovoRapid/Fiasp “PumpCart”s used for it in Europe are not yet available in Australia. Accu-Chek originally planned self-filled reservoirs for the Insight (and the pump asks you if you’re using self-filled or pre-filled) but the self-filled ones never reached production. The YpsoPump’s reservoirs can be used in the Insight (they are standard PumpCart format).
The Insight is compatible with the AndroidAPS closed-loop software, as well as the commercial Diabeloop.
Kaleido
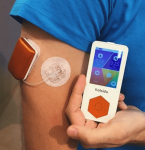 A patch pump from the Netherlands, Kaleido has a similar form factor to the now-defunct Cellnovo system, but also allows you to use longer tubing for flexibility in how you wear it. It’s the pump Diabeloop in France initially chose for their DBLG1 closed-loop system.
A patch pump from the Netherlands, Kaleido has a similar form factor to the now-defunct Cellnovo system, but also allows you to use longer tubing for flexibility in how you wear it. It’s the pump Diabeloop in France initially chose for their DBLG1 closed-loop system.
Currently I do not expect to see Kaleido reach Australia.
Tandem Mobi (t:sport)
 The t:sport has been talked about for a few years, with various prototype mock-ups seen. Here one is shown alongside the t:slim X2.
The t:sport has been talked about for a few years, with various prototype mock-ups seen. Here one is shown alongside the t:slim X2.
It apparently has a traditional “piston style” reservoir holding 200U, and no screen. It’s built to be controlled from Tandem’s t:connect app via Bluetooth. It would have the Control-IQ loop software inside it, and talk to the Dexcom G6 CGM. It would use the same infusion sets as the t:slim, and presumably new options with super-short tubing will be introduced. It’s designed to be small enough to be stuck to your skin beside the infusion site (see the image above of the similar Kaleido pump).
In December 2021 Tandem announced a name for it: “Mobi” (t:sport seems to just have been the development name). No specific timeframe has been announced, but it will be dependent on FDA approval of a remote bolus extension to the t:connect software.
They’ve mentioned a planned update for it they’ve branded “Mobi: Tubeless”, where instead of having a “pigtail” connector like the above image, the reservoir will feed directly into a housing plate that’s integrated with the cannula. So the wear experience will have some commonality with the Accu-Chek Solo (although it should be waterproof). Tandem did indicate this might come after their t:slim X3 pump.
Tandem t:slim X3
All we know about this is that it will essentially be the next generation of t:slim X2 hardware. It’s still some time away, and more detail will come to light over time.
BetaBionics iLet
 This is another not-an-actual-pump-yet. BetaBionics have been working for years on a “bionic pancreas” closed-loop system, and have conducted a number of trials in the US.
This is another not-an-actual-pump-yet. BetaBionics have been working for years on a “bionic pancreas” closed-loop system, and have conducted a number of trials in the US.
There have been two basic designs investigated: a “single hormone” (insulin-only) pump, and a “dual-hormone” (glucagon and insulin fed to two infusion sites). Insulin to push your BG down, and glucagon to bring it up: just like an actual pancreas.
BetaBionics did a lot of work with Zealand Pharma who developed dasiglucagon, an artificial glucagon which is stable in liquid form (unlike the regular glucagon in injection kits). Dasiglucagon has also recently reached the US market in a ready-to-inject emergency pen form (Gvoke).
Of course in the years all this dual-hormone development work has been going on, other single-hormone closed-loop systems have developed too. While iLet trial participants have commented about how easy the system is to use, I haven’t yet seen clinical results any better than achieved by today’s single-hormone systems. But maybe the advantage is the self-tuning nature of the system.
There are no signs yet of when an iLet product might actually appear, and trials are ongoing.
MEDISAFE WITH
 Terumo (a brand many people associate with syringes and needles) also has an insulin pump, but only within Japan.
Terumo (a brand many people associate with syringes and needles) also has an insulin pump, but only within Japan.
The “MEDISAFE WITH” is a patch pump, and will be used with the Diabeloop closed-loop system.
I’m not aware of any plans for this pump outside Japan.
DIA:CONN G8
 This Bluetooth-connected pump is from South Korea. I’m not yet aware of what countries (if any) outside Korea and China it might be marketed in.
This Bluetooth-connected pump is from South Korea. I’m not yet aware of what countries (if any) outside Korea and China it might be marketed in.
It’s advertised as coming with a phone app which is cloud-connected for sharing data with HCPs. It takes a single AA battery, holds up to 300U, and has a bolus increment of 0.05U. The delivery speed is selectable from 1-8 U/hr (in 1 U/min increments).
This pump can be used with AndroidAPS 3.0.
Sigi
 This Swiss patch pump was shown at the 2021 ATTD (Advanced Technologies and Treatments for Diabetes) conference, although it has apparently not yet been commercialised into a product. Well it looks ready, but it seems Sigi are looking for commercial partners.
This Swiss patch pump was shown at the 2021 ATTD (Advanced Technologies and Treatments for Diabetes) conference, although it has apparently not yet been commercialised into a product. Well it looks ready, but it seems Sigi are looking for commercial partners.
It has some novel features:
- Users receive two USB-rechargeable pumps to alternate between.
- Clips into and out of a cannula “plate” (which has an angled cannula).
- Uses the same PumpCart insulin vials as the YpsoPump and the Accu-Chek Insight.
- It uses Bluetooth and is designed to integrate with future loop systems.
- It administers insulin via a dual mechanism, with the PumpCart reservoir pressurising the delivery mechanism which essentially then squashes and expands a tiny section of tubing to deliver tiny parcels of insulin for accurate dosing.
Due to the compression engine I do expect that Apidra would not do well in that pump (but that’s not available in pre-filled PumpCarts anyway).
Equil
 Another patch pump, this pump seems to be looking for markets at the moment. It might be getting a little use in Europe at the moment.
Another patch pump, this pump seems to be looking for markets at the moment. It might be getting a little use in Europe at the moment.
Like the Solo, the pump has a “cannula plate” which is placed on the skin first, with the pump reservoir and base unit being connected together and then inserted into the plate. I have not seen any indication that the pump is waterproof, although it does use a different battery technology to the Solo.
The company has made some noises about a closed-loop system integrating the Equil with their AIDEX CGM, although I’m not aware that’s eventuated into actual product yet.
That’s it for now
But as usual I’m sure this page will get updated again in the coming months…