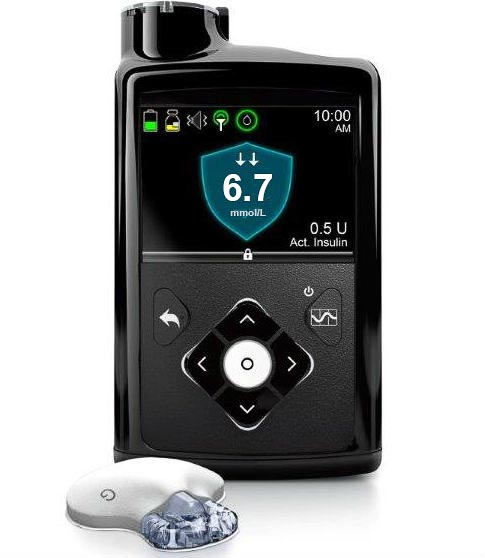
Most people interested in insulin pumps will by now be aware of the Medtronic 670G pump (sometimes mistakenly labelled as an “artificial pancreas”). This pump is the first commercial pump with basic hybrid closed-loop functionality, and was released in the US in 2017.
There has been much discussion of when the 670G will be coming to Australia. I suggest the answer may in fact be “never”.
The 6xxG family
The 640G arrived in 2015, and can talk to the Guardian 2 Link CGM transmitters to act as a CGM “receiver”. The pump has a predictive low-glucose suspend (PLGS) “SmartGuard” feature which can help protect against overnight hypoglycaemia.
Unlike the earlier 5 and 7-series “Paradigm” pumps, the radio of the 6-series pumps (to communicate with BG meters and CGM transmitters) uses the 2.4 GHz ZigBee protocol, which can be used RF-license-free worldwide. The earlier pumps had different versions using different radio frequencies (868 or 916 MHz) depending on where in the world they were sold.
So you might think that the Medtronic 6xxG models would be the same worldwide. But no.
In 2015 Australia got the 640G (later also deployed in Europe), then Japan got the 620G and the USA got the 630G in 2016. These pumps are all essentially equivalent (although they do use different BG units).
Then the 670G was introduced to the US. It uses the same BG meter, but an upgraded “Guardian 3” CGM system. It doesn’t have just PLGS, but can also increase insulin flow and forms a basic hybrid closed loop system. But like the 640G et. al. it keeps the CGM results to itself: there’s no link to phones/etc available other than via the BG meter’s USB port.
We also know there’s apparently a 690G in the development pipeline, but what features it will have are unclear.
All these 6xxG pumps looks very similar, essentially sharing the same body design.
Unofficial family members
There are also other pumps in the family:
Pumps closely related to the 670G have been used in clinical closed-loop research trials in Australia for some time. They look like the 670G but are running firmware with different algorithms (and work in mmol/l).
Now we’ve seen reports of Bluetooth being added to “a future 670G” (to be launched by April 2019). Based on Medtronic’s history, I’d be prepared to bet that this pump won’t actually be called a “670G”. Maybe it would be the 690G?
We also know another pump model was supplied to research trials in the UK and referred to as the “Encore 640G”. Apparently it was essentially a 640G with the addition of Bluetooth to connect to other trial equipment. So we know that Medtronic have implemented Bluetooth in the past.
There have presumably also been other “special” research pumps.
And so to the next pump model that Medtronic will bring to Australia. Will it have Bluetooth? And what will it be called?
650G? 660G? 670G? 680G? 690G? Personally I doubt it will be a “670G”.
And what happens whey run out of numbers?

670G..
670GX
670G X Pro
..
Are these insulin pumps, or camera models? 🙂
So long as it has an overhead cam it will be ok !
Do they have 670G in Japan yet ?
Sorry, I haven’t kept track of that.