News recently emerged about a safety recall of Medtronic insulin pumps in the US. News spread quickly across the internet, but an Australian perspective might be useful.
It’s a recall through the US Food and Drug Adminstration, and thus does not apply to Australia. The pumps affected are the 630G and 670G pumps (manufactured during specific timeframes).
If Medtronic and the Australian TGA (Therapeutic Goods Administration) bring out a recall for 640G and 670G pumps in Australia, then that will apply here. However, it is still useful to understand the recall notice.
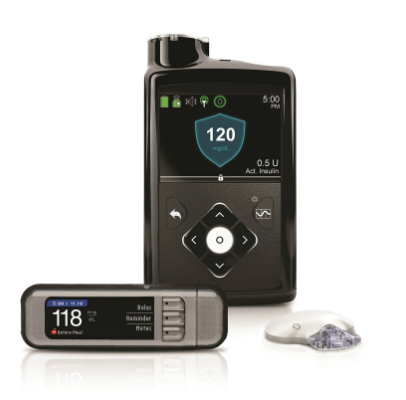
 The problem is related to the clear plastic ring at the top of the pump, which the reservoir/infusion set locks into.
The problem is related to the clear plastic ring at the top of the pump, which the reservoir/infusion set locks into.
You can just see it at the top of this image.

Apparently in recent pumps this ring is black plastic (and thus harder to see against the black body). But it still needs to be there.
The fault
The problem comes in where this ring has broken or separated from the black body, ending up sliding up and down the tubing. A result is that the reservoir is no longer locked securely in place, and insulin might not get delivered when expected (both under and OVER-delivery).
To quote from the FDA recall notice (the highlighting is mine):
The firm has received a total of 26,421 complaints in which the device malfunctioned in this manner. The firm is aware of 2,175 injuries and 1 death.
Recall instructions
Apparently Medtronic contacted affected customers last October/November and gave them the following instructions:
- Examine the retainer ring of their pump.
- Stop use of the pump and contact Medtronic for a replacement pump if the reservoir does not lock into the pump or if the retainer ring is loose, damaged, or missing. If you stop using the pump, you should follow your doctor’s recommendations and perform manual insulin injections.
- Continue using the pump if the reservoir locks in place correctly.
- If the pump is dropped by accident, check the pump and retainer ring for damage and stop use if it is damaged.
- Check the pump retainer ring and verify that the reservoir is locked correctly at every set change.
There has been no summary call to return pumps for exchange. And in fact the above instructions are reasonable things that everyone should probably have been doing anyway.
Medtronic pumps in Australia
 As I noted above, this FDA safety recall does not apply to us in Australia. However….
As I noted above, this FDA safety recall does not apply to us in Australia. However….
The symptom of a separated clear plastic ring has for years been regularly reported in Australian diabetes peer-support groups. At least on 640G pumps. The standard response has been along the lines of “Yeah, mine did that. I reported it to Medtronic and they replaced the pump.”
Here’s an image of an old 640G pump where this ring has completely gone.

As you can see, there’s still a black thread inside which the tubing connector will screw into. But it won’t be secure!
I am not aware of Australian statistics about complaints/injuries, but it would seem sensible for every user of a Medtronic 640G/670G pump to continue to keep a close eye on their devices, and contact Medtronic’s Customer Support to report any faults ASAP.
Just as you’re supposed to look over it on a regular basis to check there are no cracks/etc that would break the waterproof seal. My impression is that this has been a common issue too, and Medtronic should replace the pump.
Safety of an “approved” device
From the perspective of someone who uses opensource medical devices (e.g. my closed-loop system) another thought arises:
I’ve heard it said many times that “The official systems are safe because they’ve been approved by the regulators”. Tell that to the family of the person who died in the US.
We all need to be vigilant about the medical devices we use (no matter what they are), and hopefully know how to recognise when something isn’t quite right.

I had a 640G which got replaced about 9 times in its life and I reckon about five were for this pump ring problem. I ended up sticking it down with superglue and it never came off again — don’t know why Medtronic doesn’t use a better adhesive on it.
Thanks David for this update and the potential problem I was clueless about.
I have had a 640G and then a 670G when it received TGA approval and this is the first I have heard about this issue. I did coincidentally get an email today (3 hours after this post) from Medtronic celebrating 12 months of 670G and 4000+ T1D users averaging 72% time in range.
My 670G has a clear plastic ring (not black) which is so far secure but I will definitely check each time I change the reservoir. My pump may get more than it’s fair share of bumps knocks and drops so I should be more vidulent and this post has encouraged me to check my pump regularly.
Hopefully Medtronic will let me know to watch for this issue too.
Many thanks!
There was a mail-out by Medtronic in November to those people with pumps that Medtronic had decided were susceptible to this, giving them essentially the same instructions in this FDA recall.
So presumably Medtronic decided your pump was not at risk. But yes I do think it’s a good idea to keep an eye on the pump anyway.
I contacted medtronic about this problem with my 640G. They agreed that I should not use the pump. Then the catch…the pump is out of warranty and therefore they will not replace it. I said you have a faulty product that could cause death. Their answer…it not under warranty and there is no product recall… just a safety notice. I raised it with the TGA…thye said they will monitor the situation. I lost all faith in Medtronic as to them caring for us.