The Dexcom G6 CGM is very convenient in the way it rarely needs calibrations. However it’s not perfect. We provide a sensor code so the system knows which of 15 “batches” this sensor is part of, and this guides the auto-calibration. The transmitter takes a while for “warmup”, and then starts giving us numbers. But I have learnt to not trust those numbers too soon!
I’ve written previously about Minimizing CGM gaps, where I use two transmitters and overlap the start of one sensor with the end of the previous, and only switch my system to use the new sensor when it becomes trustworthy. I started doing this with G5, but it’s become even more important for me with G6.
In this example I have a lot of overlap. I started the new sensor early because I was concerned the old one might drop out. In fact the old one has continued to match fingerpricks and have low noise for days since! In the image shown here both CGM streams have been fed into a single Nightscout instance, where the comparisons are obvious!
The green band on this graph is 3.9-7.8 mmol/L. The red low band starts at 3.3, and the red high band starts at 10.0.
You can zoom into the above image to see the detail, but here’s some of the early wonkiness:
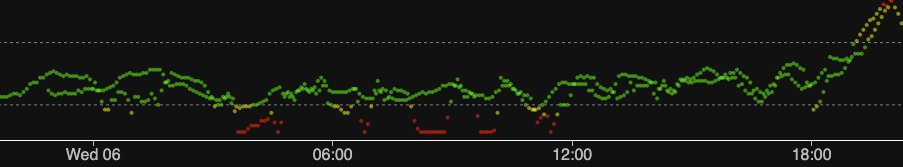
We don’t expect the sensors to produce exactly the same values (in fact the almost solid line to the right is very surprising!). But it’s taken somewhere between 13 and 30 hours for things to settle!
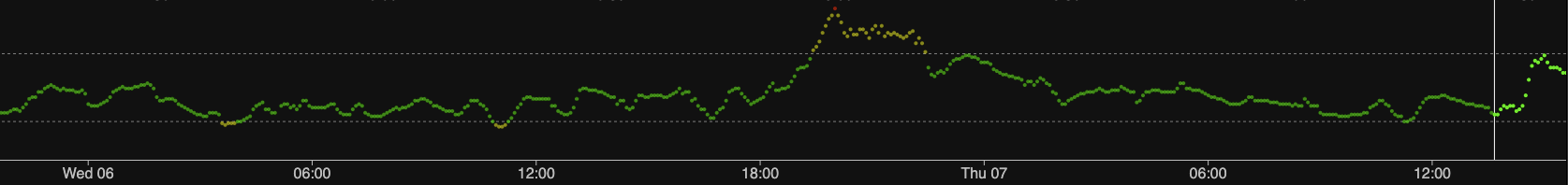
Just to be clear about which is which, here is just the old sensor.
They’re not all like this. Every now and then I get a sensor which is fairly solid from the get-go. But sometimes they take longer than this one!
If I didn’t have my dual-sensor setup, whenever I started a new sensor I would find myself having to suspend my loop from making automatic adjustments to my insulin pump for most of a day. I’d probably also have to shut off CGM alarms for a while so I could get some sleep!

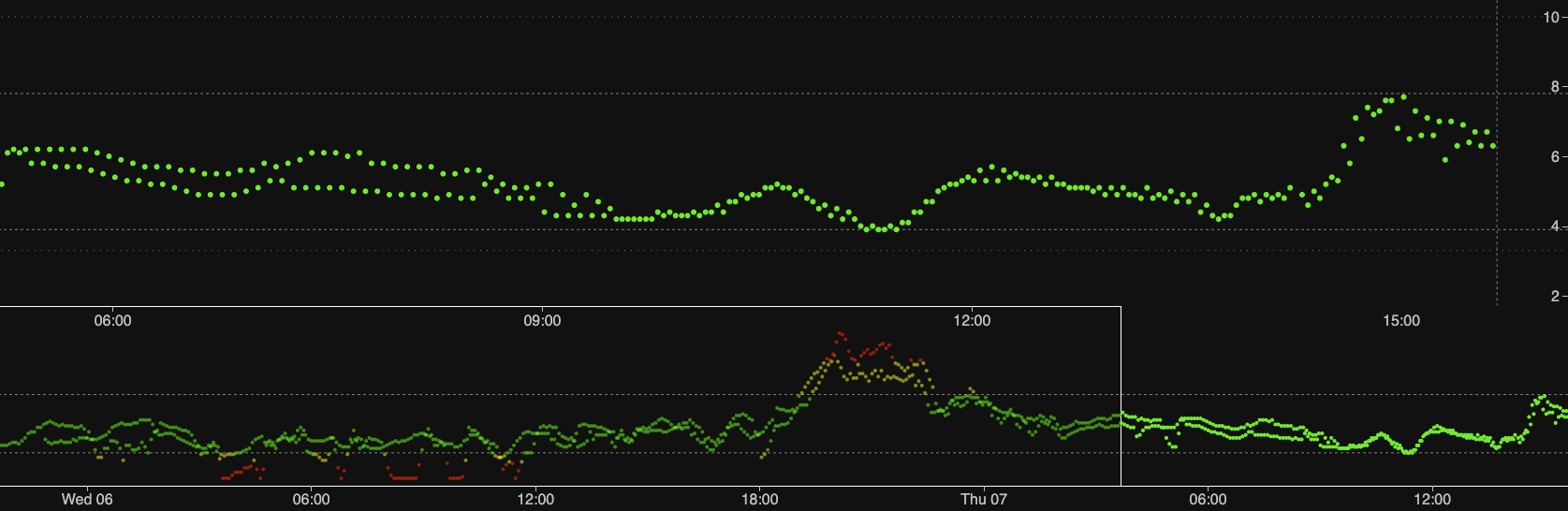
Interesting. I always finger prick my daughter after the G6 warm up session and few hours after that and the results were mostly always spot on or with insignificant difference. Could your finger prick machine need to be upgraded? The only things we noticed is a lot of “sensor errors” message when the transmitter about to die. But it always come back good again.
These graphs are comparing two G6 sensors. I didn’t spell out the fingerprick results, but they have generally matched the oldest sensor.
See all those drops in the first 12 hours for the new sensor? That’s got nothing to do with my BG meter. In fact most times a G6 sensor is quite jumpy early on.
If you’ve read some of the other articles on my site you would probably suspect I’m fairly particular about the accuracy of my meters. 🙂
Fair method to go through. I expand my smb time to every 15min in a new sensor that way eve if the new sensor is wonky it won’t necessarily start dosing with jumps but if it dips it’s only going to cut off basal for a short period.
I’m still using Libre 1’s and find much the same as you: tendencies to be jumpy at the beginning and end, but not predictable as to how long exactly they will last. I was extending them using Diabox for a while and did a lot of finger poking. At the very best I could get 18 or even 19 days, and sometimes the FSL1’s are done giving good results well before their 14 day expiry. Most of the time they make it to 14 days. It’s pretty easy to see when they are not giving good results, they are very jumpy. Same at beginning and end. If I’m on it I’ll put the next sensor in a few hours before my old one expires and just let it sit without initializing it. It seems to start off smoother if I do that.
Thanks David. Your blog posts are always very informative!
I used the Dexcom G6 for a year and had a similar experience in the first 24-36 hours, but didn’t have spare transmitters to be able to run two sensors simultaneously. I also found that the factory calibration accuracy wasn’t brilliant for me and that the sensors would often go into extended sensor error around day 7 and not last 10 days. I experimented with pre-soaking the sensors and manually calibrating, which did produce better accuracy but I still had the issues with the inconsistent sensor life, which then made it hard to pre-soak sensors!
In contrast, I’ve found that the Libre 2 with factory calibration is better (via the Libre Patched App), and depending on the sensor location can be fairly accurate on day 1. The Libre 2 has other downsides though – it’s not as responsive, even using the raw Xdrip data by the minute rather than the smoothed value and it feels “slow” to react when blood sugars are dropping fast.
Another example of the YDMV – possibly something immune system related would explain how different bodies react to different sensors.
I also have another challenge with compression lows which is another story. It does highlight how CGM is so crucial in an effective looping setup.
Thanks for your informative articles. When uploading glucose values to Nightscout from multiple sources, how do you differentiate them and what effect does multiple glucose input have on reports? Thank you.
Multiple streams in Nightscout gets very messy. I only did this to a “play” Nightscout instance for the purpose of illustrating this article.
I usually only have data from one sensor being uploaded at a time. When switching over from one to the other, the gap between two readings won’t be 5 minutes (it’s rare for two transmitters to return data in sync) but that’s about it.