NOTE: This article has been superceded by the April 2019 pump update!
There have been a few slight developments in the Australian pump landscape since my August update. So here’s a new run-down. There are 7 pumps that can currently be purchased new through private health insurance:
- Tandem t:slim X2
- Medtronic 640G
- Medtronic 670G
- Accu-Chek Combo
- Ypsopump
- Cellnovo
- DANA RS
As usual I’ll run through them with brief descriptions to give you an overview.
Where I talk about “closed-loop” systems, those are systems which use your CGM data to dynamically adjust your pump’s delivery of insulin. These are not the mythical complete “artificial pancreas” replacement, but they do add a lot of automation to help you manage your diabetes.
Medtronic 640G
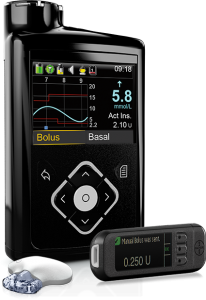 This pump integrates with Medtronic’s Guardian2 Link CGM, and uses that data for “SmartGuard” which is their predictive-low-glucose-suspend (sometimes referred to as a “hypo minimiser”) although this function is dependent on keeping the CGM calibrated and accurate.
This pump integrates with Medtronic’s Guardian2 Link CGM, and uses that data for “SmartGuard” which is their predictive-low-glucose-suspend (sometimes referred to as a “hypo minimiser”) although this function is dependent on keeping the CGM calibrated and accurate.
The pump is supplied with a linked “Contour Next Link 2.4” BG meter. The meter, pump, and CGM sensors communicate via a radio protocol which is not Bluetooth.
The reservoir in the 640G can hold up to 300U of insulin, and the basal rates can be set to 0.025 U/hr increments in 30-minute blocks.
The 640G is not compatible with any closed-loop system.
Medtronic 670G
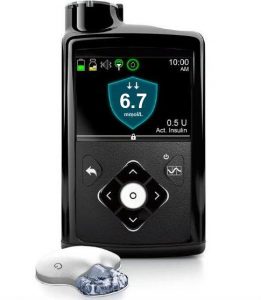 This is Medtronic’s first closed-loop system, where it will be able to vary insulin dosing (and not just suspend it like the 640G) depending on your CGM data.
This is Medtronic’s first closed-loop system, where it will be able to vary insulin dosing (and not just suspend it like the 640G) depending on your CGM data.
Although the pump started being delivered to some customers at the beginning of November, the Guardian3 CGM is not yet approved for supply in Australia. Expectations seem to be that it may be available by some time in early 2019.
In the meantime, the 670G does not have a CGM (it can’t use that of the 640G) and is a “plain” pump. You can use other CGMs such as from Dexcom, but the pump will not integrate with those. Like the 640G, this pump is not compatible with any open protocol closed-loop systems.
Because the 670G currently does not have integrated CGM, the 640G still has advantages and is being supplied, although usually with a planned transition path to the 670G. The reservoir size, insulin increments, linked BG meter, etc are the same for both pumps.
When it does get the CGM the 670G will be able to have the same SmartGuard function as the 640G, as well as having “auto mode” which is their closed-loop system. Note that it is a first-generation looping system and lacks many of the fancy features of the open protocol loop systems, although it will be one approved by the regulators.
As with any product there have been pros and cons. For example there have turned out to be various “gotchas” in use, such as when the system decides to drop out of auto mode for one of a zillion reasons you need to manually re-enable SmartGuard and not assume it’s still working.
Like all such systems it will need “care and feeding”: hopefully people don’t assume it’s going to be a “magic pill” which will fix everything for them automatically.
Accu-Chek Combo
 This pump is a relatively old design, but it seems to have proven itself as a reliable performer over the years. The Combo system is comprised of a “Spirit Combo” pump with a “Performa Combo” BG meter which is used as a remote control so the pump doesn’t have to be brought out of your pocket in normal use.
This pump is a relatively old design, but it seems to have proven itself as a reliable performer over the years. The Combo system is comprised of a “Spirit Combo” pump with a “Performa Combo” BG meter which is used as a remote control so the pump doesn’t have to be brought out of your pocket in normal use.
The Spirit can hold up to 315U of insulin, and the basal rates can be set to 0.01 U/hr increments in 1-hour blocks. The pump has a luer-lock connection for its infusion sets, so it can be used with any of the Roche/Accu-Chek sets along with the Unomedical (Animas/Tandem) infusion sets and Cleo 90.
Currently this is the pump I’m personally using, as an in-warranty pump that can be used with the AndroidAPS closed-loop system to integrate with my CGM.
Tandem t:slim X2
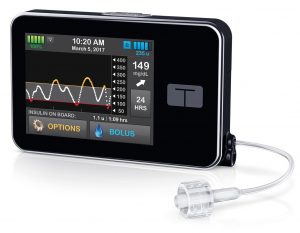 This pump is getting rolled out to two classes of user. Many Animas Vibe users are bring traded up to the Tandem (within the Vibe’s warranty period) and others are “new starts” on the pump.
This pump is getting rolled out to two classes of user. Many Animas Vibe users are bring traded up to the Tandem (within the Vibe’s warranty period) and others are “new starts” on the pump.
It’s a small pump with a touchscreen interface. The aluminium case is very robust, and gives it a slight heft in your hand. The internal battery is recharged by USB (typically topped up daily).
The insulin reservoir can hold up to 300U. The basal rate can be set with a tiny increment of 0.001 U/hr in 16 arbitrary time blocks. The pump is initially being supplied with reservoirs that have a luer-lock connection for the infusion sets. Thus they can be used with the same Unomedical infusion sets used with the older Animas pumps (along with the Accu-Chek sets for the Combo pump described above) along with the Cleo 90 set.
At some point in the future new reservoirs and infusion sets using Tandem’s new t:lock connector (which has less “dead space” than luer-lock connectors) will be introduced (the sets are made by Unomedical, and will match the existing Inset, Comfort, and Contact sets).
A feature of this pump is the ability to update its firmware at home via USB without having to have the whole pump replaced/serviced, and this will be used to add features to the pump during its 4-year warranty period. Initially the pump can act as a Dexcom G5 CGM receiver. When the Dexcom G6 is introduced to Australia (probably in early 2019) a firmware update will be released to add G6 support, and at the same time include Tandem’s “Basal-IQ” low-glucose-suspend function. This suspends and resumes basal insulin as required to keep your BG above 4.4 mmol/l. It was released to US users in August 2018, and is receiving a lot of positive feedback.
The t:slim pump is not compatible with any open protocol closed-loop system.
 Tandem are still testing the subsequent “Control-IQ” firmware update, which is their closed-loop pump control system, working to keep your BG in range all the time. Many people are understandably excited about this. Do note that even when that trial completes in 2019 there will be a lot of work still to be done to get it through the US and Australian regulators and bring it to market. Tandem have indicated it might be late 2019, but we’ll have to wait to see how good that estimate is.
Tandem are still testing the subsequent “Control-IQ” firmware update, which is their closed-loop pump control system, working to keep your BG in range all the time. Many people are understandably excited about this. Do note that even when that trial completes in 2019 there will be a lot of work still to be done to get it through the US and Australian regulators and bring it to market. Tandem have indicated it might be late 2019, but we’ll have to wait to see how good that estimate is.
AMSL have stated that the Basal-IQ and Control-IQ updates will be free in Australia (although users will presumably need to go through additional training and have medical signoff to receive the update).
The t:slim X2 is rated as IP67 waterproof, which is not quite as robust as the IPX8 rating of most other current pumps. See this article for a discussion of waterproof ratings. Although the pump is not rated quite as “proof” as an Animas Vibe, the IP67 rating does still allow for short-term immersion in water up to a metre deep.
Cellnovo
 This tiny pump is worn on the body near the infusion site, and can hold up to 150U of insulin (note that when the infusion set is replaced, the reservoir is also discarded). The basal rates can be set to 0.05 U/hr increments, in 1-hour blocks. The cannulae (both steel and teflon options) are the same as those for the Ypsopump’s Orbit sets.
This tiny pump is worn on the body near the infusion site, and can hold up to 150U of insulin (note that when the infusion set is replaced, the reservoir is also discarded). The basal rates can be set to 0.05 U/hr increments, in 1-hour blocks. The cannulae (both steel and teflon options) are the same as those for the Ypsopump’s Orbit sets.
A pair of rechargeable pumps are included (you usually swap each time you change reservoir) with a remote-control handset, and a tiny recharging base for the pumps.
Ypsopump
 The Ypsopump (from Ypsomed) is a tiny unit which links to the MyLife app on your phone via Bluetooth. Currently the app has a one-way connection: it needs the user to use the pump’s touchscreen to issue boluses, but does most of the the rest of the management and connection from the phone (including carb/bolus calculations). An update to the pump has been promised which will give the app full remote control (and probably integrate with a CGM) but we do not yet know a date for this.
The Ypsopump (from Ypsomed) is a tiny unit which links to the MyLife app on your phone via Bluetooth. Currently the app has a one-way connection: it needs the user to use the pump’s touchscreen to issue boluses, but does most of the the rest of the management and connection from the phone (including carb/bolus calculations). An update to the pump has been promised which will give the app full remote control (and probably integrate with a CGM) but we do not yet know a date for this.
The reservoir holds up to 160U of insulin, and the basal rates can be set to 0.01 U/hr increments in 1-hour blocks. The pump uses the Ypsopump-specific Orbit infusion sets (with steel and teflon options).
The reservoir is smaller than in some other pumps, although 160U is enough for many people’s requirements. Plus the reservoirs are glass (unlike the plastic of other pumps) and as such are approved for longer-term insulin storage. The tested and approved use includes the ability to pre-fill reservoirs and keep them in your fridge for up to 4 weeks. In Europe Novo supplies NovoRapid and Fiasp in PumpCart format which is compatible with the Ypsopump. We’ll see if pre-filled PumpCarts arrive in Australia at any point.
The Ypsopump cannot currently be used with any open protocol closed-loop systems, but Ypsomed have committed to working with the JDRF Open Protocol Initiative and I’m sure this option will eventually become available. Development work is ongoing.
DANA RS
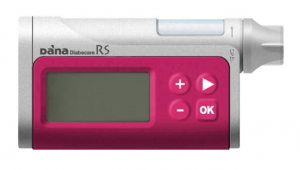
The DANA RS is in an unusual position because the supplier (Managing Diabetes) is not currently pursuing sales, although the RS is TGA-approved and like the other pumps here it’s on the list of pumps that private health insurance will purchase.
It’s primarily being sold to people who are seeking it out because of its official support for the AndroidAPS open protocol loop system.
The DANA RS has a 300U insulin reservoir, and basal rates can be set to 0.01 U/hr increments in 1-hour blocks. By default it uses DANA-specific infusion sets (including a teflon one made by Ypsomed and equivalent to the OrbitSoft set).
That’s it for now
Hopefully our pump options will continue evolve over time, so expect an update to this review in 2019.
For anyone choosing a pump with a view to having a closed-loop system, check out my November article about closed-loop pump choices.


Dana is a pump that I would not recommend. Trying to get support, especially when it had a problem was a real pain as it took weeks to get a loan pump from the supplier, who said it was my fault that the pump had stopped working, and mine was sent back to Korea where it took 4 months to have it repaired and returned. My Dana is sitting in its box and has been there for nearly 3 years.
I am amazed that the amount of work involved in wearing any of these pumps with all of the latest technology limits the use to tech smart individuals, more monitoring and awareness than I ever had to deal with while wearing my smaller older pump and even on needles when I was maintaining excellent control with several needles a day. In my 64 years of using insulin, I must comment that I cannot see many positive benefits – have to daily recharge, have to link to this and that. I have spent my life controlling this disease and looking at all of these gadgets, I am experiencing a feeling of the disease and its associated technology controlling me.
I have you heard ny updates on the Dexcom G6
No news on G6 timeline yet. It seems to be still waiting for approval from the TGA.
Any News yet on Dexcom G6, it feels like a total Blackout,,,,
we are planning to have TSlim with G6 for my son in August 2019, with Dexcom G6 in Australia market yet I will be stuck with 670 and really don’t want to do that, can anyone share the good news.
The distributor (AMSL) can not yet legally even talk about the Dexcom G6, as it’s still waiting for the TGA to approve it as a therapeutic device for Australia. Once the TGA eventually (we expect!) approves it, I expect things will move quickly. And hopefully the Basal-IQ update for the t:slim (to allow it to use the G6) is also in the approval pipeline.
Note that there is a significant medical conference in August which I’m sure many companies will use as a platform to launch new products. I’m sure the TGA doesn’t feel pressure to meet anyone else’s marketing deadlines though.