NOTE: This article has been superceded by the December 2020 update.
There have only been a few changes in the Australian pump landscape in the 6 months since my last update. Expect some more changes in the coming months, but here’s the latest run-down.
There are currently 5 pumps that can be purchased new through private health insurance:
There are also some older pumps still in use, such as:
The consumables for the above pumps are available through NDSS. The reservoirs for the Animas Vibe are no longer available, although there are probably some people still working through their stockpiles.
As usual I’ll run through them all with brief descriptions. Then I’ll mention other pumps we’re aware of which are not available. Some of them (such as the Medtronic 770G) might be available very soon.
For new pumps to be available in Australia, the first hurdle they need to pass is registration with the Therapeutic Goods Administration (TGA), after which doctors and suppliers are legally allowed to talk about them. The next hurdle is generally getting listing on the Federal Department of Health’s Prostheses List so they’re eligible for supply by health insurance. The Prostheses List was just updated: effective July 2020.
A note about “closed-loop” systems, those are systems which use your CGM data to dynamically adjust your pump’s delivery of insulin. These are not the mythical complete “artificial pancreas” replacement, but they do add a lot of automation to help you manage your diabetes. For information about the open protocol closed-loop systems, see my separate page on the Australian options.
Pumps available new in Australia
The following pumps are available new. Usually they’re bought through private health insurance, where they’re classified as prosthetic devices. Not all private health policies cover these: check with your own insurer as to coverage. But if they cover “pumps” then they’ll cover all of these.
Accu-Chek Combo
 This pump is a relatively old design, but it has proven itself as a reliable performer over the years. The Combo system is comprised of a “Spirit Combo” pump along with a “Performa Combo” BG meter which is used as a remote control so the pump doesn’t have to be brought out of your pocket in normal use.
This pump is a relatively old design, but it has proven itself as a reliable performer over the years. The Combo system is comprised of a “Spirit Combo” pump along with a “Performa Combo” BG meter which is used as a remote control so the pump doesn’t have to be brought out of your pocket in normal use.
The Spirit Combo can hold up to 315U of insulin, and the basal rates can be set to 0.01 U/hr increments in 1-hour blocks. The pump has a luer-lock connection for its infusion sets, so it can be used with any of the Roche/Accu-Chek sets along with the Unomedical (“Animas”) infusion sets, the Cleo 90 and Medtronic’s Quick-set.
Currently this is the pump I’m personally using, as an in-warranty pump that can be used with the AndroidAPS closed-loop system to integrate with my CGM.
Accu-Chek Solo
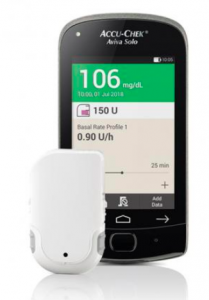 This tubeless patch pump has multiple components, starting with a cannula patch onto which the pump clips. The cannula is 90˚ teflon with two length options. There’s a disposable 200U reservoir which also includes the battery which powers the body of the pump. The pump body nominally lasts 4 months. The Aviva Solo remote controller has an integrated BG meter (although you can enter corrections using a BG value you’ve retrieved from another meter or CGM).
This tubeless patch pump has multiple components, starting with a cannula patch onto which the pump clips. The cannula is 90˚ teflon with two length options. There’s a disposable 200U reservoir which also includes the battery which powers the body of the pump. The pump body nominally lasts 4 months. The Aviva Solo remote controller has an integrated BG meter (although you can enter corrections using a BG value you’ve retrieved from another meter or CGM).
 The Solo pump is not waterproof: you are expected to remove it (leaving the cannula patch in place) for swimming and showers. This is primarily because the battery has to use a “zinc-air” chemistry to get enough capacity, and without access to air the battery stops working.There’s a small vent on the outside of the pump, that if you covered with tape would kill the pump.
The Solo pump is not waterproof: you are expected to remove it (leaving the cannula patch in place) for swimming and showers. This is primarily because the battery has to use a “zinc-air” chemistry to get enough capacity, and without access to air the battery stops working.There’s a small vent on the outside of the pump, that if you covered with tape would kill the pump.
Because the pump body lasts for 4 months, when you buy the pump each year you will receive three bodies. Plus if it’s the first time you’ve bought the pump you’ll receive a handset.
In 2019 I wrote about my experience wearing a dummy Solo.
Tandem t:slim X2
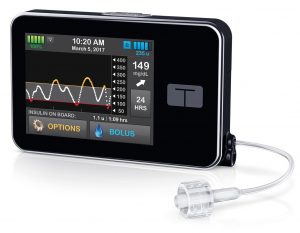 This pump is a small pump with a touchscreen interface. The aluminium case is very robust, and gives it a slight heft in your hand. The internal battery is recharged by USB (typically topped up at least every few days).
This pump is a small pump with a touchscreen interface. The aluminium case is very robust, and gives it a slight heft in your hand. The internal battery is recharged by USB (typically topped up at least every few days).
The insulin reservoir can hold up to 300U. The basal rate can be set with a tiny increment of 0.001 U/hr in 16 arbitrary time blocks. However, the minimum basal rate is 0.100 U/hr (or 0), and this can cause issues for some paediatric use.
Note that even if your basal rate is 0.6 U/hr, that minimum 0.1 still applies to any temp basal. Thus the lowest TBR you’d be able to apply would be 17%. Not 10%. If your basal rate was 0.3 U/hr you’d only be able to drop to 34%.
The Tandem reservoirs are compatible only with the Tandem t:lock infusion sets. Some people are still using the previous reservoirs that could be used with any luer-lock infusion set, but those have been discontinued.
Note that Apidra insulin is not compatible with the Tandem reservoirs (it’s not officially listed for pump use in Australia, but is sometimes used in other pumps). Apidra fails catastrophically in this pump. Fiasp is officially not supported either, although some people apparently do use it successfully.
A touted feature of this pump is the ability to update its firmware at home via USB without having to have the whole pump replaced/serviced, and this will be used to add features to the pump during its 4-year warranty period. Right now the pump can act as a Dexcom G5 CGM receiver.
Now the Dexcom G6 has been introduced to Australia (June 2020) hopefully soon Tandem’s “Basal-IQ” firmware update will get TGA approval and become available. That will enable support for the G6 (but remove support for the G5). Basal-IQ has a low-glucose-suspend function that suspends and resumes basal insulin as required to keep your BG above 4.4 mmol/l. It was released to US users in August 2018, and has received a lot of positive feedback. It’s a lot finer-grained than the cruder suspend function used in Medtronic’s 640G pump.
 Tandem’s subsequent “Control-IQ” firmware update, which is their closed-loop pump control system, should come at some point, bu no-one’s got a hold on when. It was released in the US at the end of 2019, but no-one knows about Australia. I’m not expecting it in 2020. AMSL have stated that both the Basal-IQ and Control-IQ updates will be free in Australia (although users will presumably need to go through additional training and have medical signoff to receive the update). As long as your pump is still in warranty.
Tandem’s subsequent “Control-IQ” firmware update, which is their closed-loop pump control system, should come at some point, bu no-one’s got a hold on when. It was released in the US at the end of 2019, but no-one knows about Australia. I’m not expecting it in 2020. AMSL have stated that both the Basal-IQ and Control-IQ updates will be free in Australia (although users will presumably need to go through additional training and have medical signoff to receive the update). As long as your pump is still in warranty.
The t:slim X2 is rated as IP67 waterproof, which is not quite as robust as the IPX8 rating of most other current pumps. See this article for a discussion of waterproof ratings. Mind you, the IP67 rating does still allow for short-term immersion in water up to a metre deep.
The t:slim pump is not compatible with any open protocol closed-loop system.
YpsoPump
 The YpsoPump (from Ypsomed) is a tiny unit which links to the MyLife app on your phone via Bluetooth. Currently the app has a one-way connection: it needs the user to use the pump’s touchscreen to issue boluses, but does most of the the rest of the management and connection from the phone (including carb/bolus calculations).
The YpsoPump (from Ypsomed) is a tiny unit which links to the MyLife app on your phone via Bluetooth. Currently the app has a one-way connection: it needs the user to use the pump’s touchscreen to issue boluses, but does most of the the rest of the management and connection from the phone (including carb/bolus calculations).
It seems a series of updates are in the pipeline for the YpsoPump. Integration of Dexcom CGM data into the MyLife app. A pump update that supports bolusing directly from the app, and another update that lets the pump be controlled from a closed-loop controller. Ypsomed have an agreement with Dexcom (who own the TypeZero algorithm) and one option should come from that. But timelines for those things are not yet known.
The reservoir holds up to 160U of insulin, and the basal rates can be set to 0.01 U/hr increments in 1-hour blocks. The pump uses the Orbit infusion sets (with steel and teflon options) although more options may be introduced at some point.
The reservoir is smaller than in some other pumps, although 160U is enough for many people’s requirements. Plus the reservoirs are glass (unlike the plastic of other pumps) and as such are approved for longer-term insulin storage. The tested and approved use includes the ability to pre-fill reservoirs and keep them in your fridge for up to 4 weeks.
The YpsoPump cannot currently be used with any open protocol closed-loop systems, but development work is ongoing.
Medtronic 670G
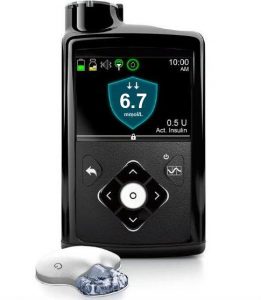 The 670G pump was Medtronic’s first closed-loop system. The pump acts as the receiver for the Guardian3 Link CGM, which it needs for the closed-loop functionality.
The 670G pump was Medtronic’s first closed-loop system. The pump acts as the receiver for the Guardian3 Link CGM, which it needs for the closed-loop functionality.
The pump is supplied with a linked “Contour Next Link 2.4” BG meter. The meter, pump, and CGM sensors communicate via a radio protocol which is not Bluetooth.
The reservoir in the 670G can hold up to 300U of insulin (it can also use a 180U reservoir), and the basal rates can be set to 0.025 U/hr increments in 30-minute blocks.
The Guardian3 CGM has sensors that last for 7 days, and require calibration within every 12 hours.
With its CGM the 670G is able to have the same SmartGuard function as the 640G, as well as having “auto mode” which is their closed-loop system. Note that it is a first-generation looping system and lacks many of the configuration options and features of the open protocol loop systems, although it is approved by the regulators and available without you having to assemble it yourself. The 670G is not compatible with any external closed-loop systems.
Like all loop systems it needs “care and feeding”: hopefully people don’t assume it’s going to be a “magic pill” which will fix everything for them automatically.
Other pumps in use in Australia
While not available new, the following pumps are still in use by many people.
Medtronic 640G
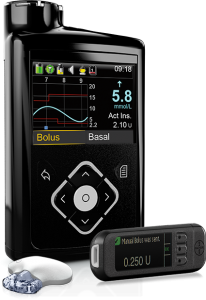 This pump originally integrated with Medtronic’s Guardian2 Link CGM, but also supports the Guardian3 Link that was introduced with the 670G.
This pump originally integrated with Medtronic’s Guardian2 Link CGM, but also supports the Guardian3 Link that was introduced with the 670G.
It uses that data for “SmartGuard” which is their predictive-low-glucose-suspend (sometimes referred to as a “hypo minimiser”) although this function is dependent on keeping the CGM calibrated and accurate.
The pump is supplied with the same BG meter as the 670G, and the reservoir capacity and consumables are the same.
The 640G is not compatible with any closed-loop system.
Medtronic Paradigm “Veo” (554 or 754 models)
 These were Medtronic’s previous generation of pumps.
These were Medtronic’s previous generation of pumps.
The reservoir in the 554 can hold up to 180U of insulin, and the 754 can hold up to 300U (or use the 180U option). The basal rates can be set to 0.025 U/hr increments in 30-minute blocks.
The pumps support Medtronic’s old “Enlite” CGM system (uses the same sensors as the Guardian2 CGM, but a different transmitter) and they have a primitive low-glucose-suspend function (which suspends when you get to the boundary point, not when the pump predicts you’re soon going to get there).
These pumps were originally rated with IP67 dust/waterproofing, but especially given their age I would not regard any of them as waterproof today.
The latest firmware versions of these pumps are not compatible with any open protocol closed-loop systems. Older versions with firmware up to 2.7A are “loopable” and supported by both the Loop and OpenAPS closed-loop systems. As are many other older Paradigm models.
Animas Vibe
 Animas shut down and discontinued this pump (back in early 2018). The transition took a while, and supply of reservoirs lasted until February 2020.
Animas shut down and discontinued this pump (back in early 2018). The transition took a while, and supply of reservoirs lasted until February 2020.
The Vibe reservoir can hold up to 200U, and the basal rates can be set to 0.025 U/hr increments in 30-minute blocks. The pump has an integrated receiver for the Dexcom G4 CGM system. The Vibe is NOT compatible with any closed-loop systems.
DANA R and RS
 The DANA R and RS pumps look almost identical. The main differences are the R’s use of Bluetooth 2 rather than the lower-power Bluetooth LE, and a correspondingly-worse battery life (approximately a third of the lifespan).
The DANA R and RS pumps look almost identical. The main differences are the R’s use of Bluetooth 2 rather than the lower-power Bluetooth LE, and a correspondingly-worse battery life (approximately a third of the lifespan).
The pumps have a 300U insulin reservoir, and basal rates can be set to 0.01 U/hr increments in 1-hour blocks. They use DANA-specific infusion sets (including a teflon one made by Ypsomed and equivalent to the OrbitSoft set). The OrbitMicro steel cannulae (available on NDSS without tubing) can be used with the DANA tubing if you need to use those. The special batteries for the pump are supplied in each box of reservoirs.
The supplier (Managing Diabetes) has ceased sales of the RS, but is still supporting current users. They may be looking to the upcoming DANA-i, but won’t be able to talk about it until it has TGA approval.
Both pumps are supported by the AndroidAPS closed-loop system.
A non-T1D pump
V-Go
 The V-Go is available in Australia, but is designed for use in Type 2 diabetes, not Type 1. It is a simply mechanical (spring-driven) device for 1-per-day use, with a fixed infusion rate. I have previously written about the V-Go.
The V-Go is available in Australia, but is designed for use in Type 2 diabetes, not Type 1. It is a simply mechanical (spring-driven) device for 1-per-day use, with a fixed infusion rate. I have previously written about the V-Go.
Other insulin pumps
That’s it for the current Australian pump options, but there are some other pumps around the world which Australian folk keep asking about.
Medtronic 770G
 This pump is new. Medtronic have not made announcements about it yet, but it has received TGA approval and is listed on the July 2020 update to the Prostheses List.
This pump is new. Medtronic have not made announcements about it yet, but it has received TGA approval and is listed on the July 2020 update to the Prostheses List.
From information out of the recent ADA conference in the US it seems to use the hardware of the upcoming 780G pump (see below) but uses the algorithm of the 670G. It looks very similar to the 670G, as the basic body design is shared with everything back to the 640G. It will have a few additional features, and apparently when the 780G reaches the market may be upgradable to the 780G without needing a new pump.
But exactly what features the 770G will have in Australia remains to be seen. Apparently it has Bluetooth, but what functionality will be available in any phone app is unknown.
Medtronic 780G
This is Medtronic’s touted next closed-loop pump. It (or parts of it) are being trialled at some locations around the world. It apparently has Bluetooth for connecting to your phone, but the thing most people talk about is a more-advanced closed-loop algorithm than the 670G. The 770G pump described above seems to be effectively an early release of it.
DANA-i
 This will be the next pump from SOOIL. It has been shown at some trade shows so far, but has not reached markets yet. It’s being targetted at regulatory approval in the US and Europe. It can be seen as the next generation of the RS. It uses the same reservoirs and infusion sets, but uses a standard AAA battery.
This will be the next pump from SOOIL. It has been shown at some trade shows so far, but has not reached markets yet. It’s being targetted at regulatory approval in the US and Europe. It can be seen as the next generation of the RS. It uses the same reservoirs and infusion sets, but uses a standard AAA battery.
The DANA-i is another of the pumps that will be aiming at use with commercial closed-loop systems. I gather that unlike the earlier R and RS pumps the manufacturer will not be supporting its use in opensource software such as AndroidAPS.
Insulet OmniPod
 This tubeless patch pump is available in the US and Europe, but although medically it’s been approved for use in Australia since 2012 it has never been sold here. The economic model simply doesn’t fit the funding model used in Australia.
This tubeless patch pump is available in the US and Europe, but although medically it’s been approved for use in Australia since 2012 it has never been sold here. The economic model simply doesn’t fit the funding model used in Australia.
Every 3 days a whole new pump is required: the only permanent piece is the “PDM” remote controller. This would shift most of the cost from your health insurance to the NDSS which supplies consumables, and no-one’s been able to work out a compromise. It also produces a lot of hard-to-recycle waste. But the pump can be worn during swimming/showering/etc. If the pump detaches, it can’t be re-attached.
Incidentally, the old version’s internal name is “Eros”, and uses 433 MHz radio to link the PDM and the pumps. Their new “DASH” system instead uses Bluetooth but it otherwise the same. The Eros OmniPods can be used with opensource closed-loop systems (primarily Loop at the moment). The DASH will be used by Tidepool’s future commercial closed-loop version of Loop, as well as Insulet’s own Horizon closed-loop.
Accu-Chek Insight
 This pump is used in Europe, but not available here (although it was registered with the TGA back in 2015, as well as added to the Prostheses List). It uses essentially the same cartridges as the YpsoPump, but the pre-filled Novorapid “PumpCart”s used for it in Europe are not available in Australia. The end of the pump is actually part of the infusion set tubing (the pump needs its own collection of infusion sets). The Insight is supported by the AndroidAPS closed-loop software.
This pump is used in Europe, but not available here (although it was registered with the TGA back in 2015, as well as added to the Prostheses List). It uses essentially the same cartridges as the YpsoPump, but the pre-filled Novorapid “PumpCart”s used for it in Europe are not available in Australia. The end of the pump is actually part of the infusion set tubing (the pump needs its own collection of infusion sets). The Insight is supported by the AndroidAPS closed-loop software.
I do not expect to see the Insight appear in Australia.
EOFlow patch pump
 This patch pump from Korea got some press in March 2019 when it got “Breakthrough Device” designation from the US FDA. However it is still a long way from market. Development plans apparently include integration with the POCtech CGM into a single unit.
This patch pump from Korea got some press in March 2019 when it got “Breakthrough Device” designation from the US FDA. However it is still a long way from market. Development plans apparently include integration with the POCtech CGM into a single unit.
Medtrum A6
 This tubeless patch pump from China has had TGA registration for some time, but does not seem to be on anyone’s marketing plans. Reports from overseas users characterise it as a relatively primitive device (especially its controller device).
This tubeless patch pump from China has had TGA registration for some time, but does not seem to be on anyone’s marketing plans. Reports from overseas users characterise it as a relatively primitive device (especially its controller device).
Currently I do not expect to see the Medtrum reach the Australian market.
Kaleido
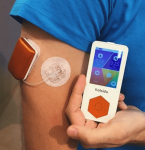 Another European patch pump, Kaleido has a similar form factor to the Cellnovo system, but also allows you to use longer tubing for flexibility in how you wear it. It’s the pump Diabeloop in France chose for their DBLG1 closed-loop system (which did use Cellnovo during development).
Another European patch pump, Kaleido has a similar form factor to the Cellnovo system, but also allows you to use longer tubing for flexibility in how you wear it. It’s the pump Diabeloop in France chose for their DBLG1 closed-loop system (which did use Cellnovo during development).
Currently I do not expect to see Kaleido reach Australia.
That’s it for now
I’m sure our pump options will continue to evolve, so expect more updates to come!


Hey David, small correction if I may – Sooil has said they will open the Dana-i to the Open Source community in the same way as the Dana R and the RS. We can look forward to seeing it supported in a future version of Android APS 🙂
With the t:slim it looks like it bluetooth connects to a mobile phone but only to show recent data, not to control the pump, change basals and make boluses – like how the Android APS app makes a mobile device a remote control for the Accu-chek Spirt Combo. Is that correct? Is there a third party app or another way to remotely access and use the t:slim?
Not it doesn’t.
The most recent pump firmware available in the US does support their new t:connect phone app. But there are no 3rd-party options yet.
And neither that pump firmware function or the t:connect app are available in Australia.
thank you David for all your hard work and insights. It is much appreciated and it has given me much food for thought