The 2023 Fiasp supply dramas are entering a new phase.
We have an opportunity to contribute to the process in the next week. Scroll on and you’ll find details. But first, some background. Or jump ahead if you’re in a hurry….
History recap
In June 2019 Fiasp was added to the Australian Pharmaceutical Benefits Scheme (PBS). Fiasp is “faster insulin aspart”, and has a greatly accelerated action time compared to the usual insulin aspart (NovoRapid). Insulin lispro (Humalog) and insulin glulisine (Apidra) are the other rapid insulins currently available in Australia, and they have very similar timing to NovoRapid. Fiasp is the only “ultra-rapid” insulin that has been available to Australians.
Managing insulin injections/pumping, and lining up the action time of the insulin with that of food and exercise is a balance that we are constantly working with.
In the years since, Fiasp has allowed many people to keep that balance on track, making life much easier. In 2023 we were told that about 15,000 people were relying on Fiasp.
In February 2023 we heard that Fiasp was about to be delisted from PBS. The March PBS update showed it was to become unavailable on April 1.
This was apparently due to a price dispute between the Australian Government and Novo. Essentially, PBS was reducing how much they wanted to pay for insulins, and even apparently lowered their offer for Fiasp even further than NovoRapid. There’s evidence of discussion at PBAC (the PBS Advisory Committee) that basically indicated it wouldn’t matter if Fiasp wasn’t available, as it was essentially “just another insulin aspart”.
Those of us who’ve used Fiasp know that it’s usually significantly different in speed of action compared to NovoRapid, and that this speed can have a significant impact on us.
In mid-March 2023 the Federal Health Minister issued a Supply Order to Novo, resulting in Fiasp being in “Supply Only” state for 6 months (up until the end of September 2023). This means that new PBS prescriptions for it could not be written after March, but existing prescriptions could still be filled.
The current setup has no access for many people at the moment, and no access for anyone by October 2023!
So who does this affect?
So currently there are multiple populations:
- People who have current Fiasp prescriptions and are able to access it (until they use up those prescriptions or October runs around).
These people are obviously worried about what their options will be at that point. - People who were using Fiasp but were not able to get new prescriptions in the few weeks of warning we had. Not everyone is watching this stuff all the time.
These people are now either just using up their remaining supply, or are already having to step back to slower insulins to manage their diabetes.
As mentioned above, that’s about 15,000 people. But sadly, there are also people who didn’t know that Fiasp existed until it was taken away.
There are of course people who tried Fiasp and found it didn’t work for them, and people who have been simply sticking with their older insulins (there are many understandable reasons behind this). Apart from anything else, there can be a lot of “inertia” involved in managing a chronic condition like diabetes.
Fiasp formats
Yesterday’s article about insulin containers (“Re-usable insulin pens”) is relevant here. Please read it now if you haven’t already.
One of the things we moaned about in 2019 was the fact that Fiasp was not available in Penfill format. Only in vials and in disposable pens. But we coped.
But those formats have this year been subject to unsuccessful PBS price negotiations (see above). Now, there may be a new hope.
The agenda for the upcoming July meeting of the PBAC (Pharmaceutical Benefits Advisory Committee) was updated this month with a few new items. One in particular looks interesting:
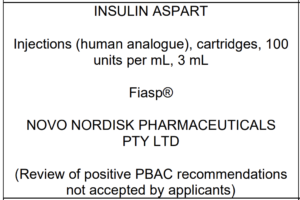
It seems as though there’s a chance that Fiasp in Penfill form could be added to PBS. I don’t know all the departmental negotiation over prices and politics around this, but it does for example seem to open up the possibility of access without needing someone to reverse a previous decision. Fiasp in Penfill form was submitted to PBAC years ago, so this would just be re-opening that case.
Using Penfills
 You may not have used Penfills before, but as explained in yesterday’s article they can be easily used for both pen injections and for filling pumps. If we end up only being able to access Fiasp in Penfill format that might not be ideal for everyone, but it’s a whole lot better than not being able to access it at all!
You may not have used Penfills before, but as explained in yesterday’s article they can be easily used for both pen injections and for filling pumps. If we end up only being able to access Fiasp in Penfill format that might not be ideal for everyone, but it’s a whole lot better than not being able to access it at all!
What we can do to help!
![]() PBAC is currently accepting comments and submissions on the items on the July agenda. Submissions close on May 24, which means we have just over a week available to us!
PBAC is currently accepting comments and submissions on the items on the July agenda. Submissions close on May 24, which means we have just over a week available to us!
As consumers it can’t hurt the process to make PBAC conscious of the impact Fiasp has for us. Then we can just hope that our submissions add to the evidence that the committee will be considering, and that a useful outcome will appear before October!
Someone’s making this harder than it needs to be
The standard process to provide feedback is straightforward: they have a web form to fill out. One of the fields is a pull-down list of which medicine you wish to comment on.
However, “INSULIN ASPART” can not be selected! At least one other medicine on this agenda has a similar issue.
The Department has not responded to repeated requests to have the form fixed. So the web form seems to be useless for us.
How to make submissions
We can make submissions by email (to commentsPBAC@health.gov.au). But this is a free-form process, so you need to be careful to submit all the required information. Essentially we need to reproduce the web form:
| Your name | |
| Your email address | |
| Your phone number | |
| What state you live in | |
| Consent | That you consent to the content of your submission being published (discussed further below). |
| The medicine you’re commenting on | “INSULIN ASPART (Fiasp)” |
| Your category.
One of: |
There are some more categories spelt out on the web form, but those would not apply to most of us. |
| How did you find out about the consultation | Feel free to drop this website name, but “Community advocacy” seems quite an appropriate answer. |
| Your comments, answering these 5 questions.
Try to follow these guidelines from the department. |
Q1: Outline your experience with diabetes. “What is the impact of your health condition on your life? Try to be as specific as possible including impacts on your everyday activities, work, family, friends, mental and emotional health.“ |
Q2: How is the medical condition currently treated?
|
|
Q3: What do you see as the advantages of this proposed medicine for you?
After you’ve spelt out the advantages for you of Fiasp, I would think that retaining access to any format of Fiasp would be a significant advantage of the item being discussed by the committee! |
|
Q4: What do you see as the main disadvantages of this proposed medicine?
|
|
| Q5: Any additional comments. | |
| Conflicts of interest | Presumably do you not own shares in Novo Nordisk, but whatever your situation you do need to make a declaration. |
You can attach a PDF or Microsoft Word document to your submission if it helps. As long as it’s no larger than 25 MB.
The department actually provides a Word document template to make things a little easier. You can download it, insert “INSULIN ASPART (Fiasp)”, and start filling in details.
Make your submission count!
The above details may seem like a long process, but please don’t ask for a sample set of answers for you to submit.
The department is strict about things that are not acceptable.
If you just copy someone else’s submission and put in a form letter, it will be rejected.
Other things to consider when writing
This is quoting from the web form:
- All input from individuals will be made available in summary form to the sponsor of the application and the PBAC.
- No identifying information about individuals or third parties will be included in the summary. This type of information will be removed by the Department.
- The PBAC may also have access to de-identified individual responses.
- All input from groups or organisations will be provided in full to both the PBAC and its subcommittees and the sponsor of the medicine. Any identifying information relating to third parties detected will be removed prior to distribution.
- In addition, all input received will be noted in the relevant Public Summary Document. Public Summary Documents are available approximately four (4) months after the PBAC meeting and outline the PBAC discussion and advice.
You should confirm in your submission that you consent to your submission being used that way.
So:
- As individuals your submissions will be summarised. Make it concise and sweet, folks!
- Don’t include identifying personal details (except for the name/email/phone items). Give them less to delete.
- Four months after the July meeting would be November. While there’s a chance that even if the PBAC’s recommendations are positive then it might not be enacted until after the current Fiasp arrangement expires, but somehow I doubt that the Health Minister (Mark Butler) would make that political mistake. We hope that if he receives a positive recommendation from PBAC that he will act on it promptly.
It would not be setting a new precedent for the government to enact things before they documented them…
Speak up now, or…
You only have until May the 24th to get your voice added to the official record. Don’t delay!


Thanks David for this. They don’t make it easy for us.
Thanks David – really appreciate your support as I would have got lost if I had just gone to the website.
Thankyou so much for this information David, we need to make every submission count and this will ensure it does,
Great summary and a clear call to arms. I’m drafting my submission now.
The agenda item looks slightly strange in the circumstances of the other Fiasp types are in the process of being removed from the PBS.
Do i understand the item correctly in the applicant is the same as sponsor and in this case is Novo? ie Novo wouldn’t supply Fiasp cartridges even though was PBAC approved
The prefilled pens retail for 5-10% more than cartridges, so swapping universally to cartridge only would save the Government a reasonable amount of money. For Fiasp think price difference is approx $10 per prescription x 15k users at say 1.5 prescriptions of Fiasp(complete guess!) per year is approx $225k saved.
(Note in above Fiasp vials are at best only a few percentage points of use, disposable pens is high 90s)
Upscale this to all the insulin types for type 1’s would supercharge the savings. Apparently approx 150k type 1 diabetics in Australia at say 3 insulin prescriptions per year by a $7.50 saving per prescription by 75% (percentage of scripts that are disposable pens) gives a saving to Medicare of $2.5m dollars.
Above ignores Type 2 users so saving is probably bigger
Can understand now why the sales reps are pushing doctors so hard to preferentially script the disposable pens
I think you’re ignoring a significant factor.
My understanding of why Fiasp Penfill wasn’t available through PBS was a cost decision. There are statutory limitations on the number of forms of a medication (“insulin aspart” in this case) they can have on PBS without incurring a price cut across all forms. Given that there are a number of other forms of insulin aspart already listed, do you think Novo would like a 10% cut on all of them?
Yes profit margins presumably came into play in determining which form they were going to cut.
With two forms (Fiasp vials and pens) gone, they’ve got room for another…
Thanks David for clearly outlining the process of sending our submission to PBAC. I’ve done mine this evening by downloading the editable “Hard Copy” version of the form from the website link below, added INSULIN ASPART (Fiasp) as it’s not listed on the Hard Copy either, saved the completed form and emailed it to the address you provided.
Another thing I noticed when completing the “Hard Copy” form provided on the PBAC website; there is a compulsory consent section to respond to in order for the submission to be accepted. Just be aware of that if copying and pasting the ‘free-form’ template offered in the above article. You will need to cover the consent section in order for your submission to be accepted.
https://ohta-consultations.health.gov.au/ohta/pbac-july-2023/supporting_documents/Hard%20copyPBAC%20Consultation%20SurveyJuly2023%20Meeting.docx
Thanks so much for your great info.
I wrote to PBAC to clarify what is actually being discussed in the Fiasp agenda item at the July meeting and received the reply I’ve pasted below. Please anyone planning to make a submission read this before you do so otherwise your comments may be irrelevant. Key points we need to make:
1. strongly encourage Novo Nordisk to make the penfills available as an alternative to the two preparations that we delisted earlier this year.
2. Ensure the government offers a fair price
3. Ensure the government understands that Fiasp is the only ultra fast acting insulin available in Australia, and they have made a significant error grouping it with novorapid and the like which are not comparable.
Pasted response from pbac@health.gov.au:
Thank you for your email regarding the July 2023 Pharmaceutical Benefits Advisory Committee (PBAC) agenda which includes a review of positive recommendations for Insulin aspart (Fiasp®) injections (human analogue), cartridges, 100 units per mL, 3mL (also known as Fiasp Penfill®). Please note that this review item does not relate to the current Pharmaceutical Benefits Scheme (PBS) listings of Fiasp® insulin aspart 100 units/mL fast acting injection, 1 x 10 mL vial, and Fiasp® FlexTouch insulin aspart 100 units/mL fast acting injection, 5 x 3 mL pen devices. The continued listing of these medicines on the PBS are a separate matter and the Government is committed to working with diabetes advocacy groups and industry to ensure Australians living with Type 1 diabetes will continue to have access to affordable and effective insulin treatments on the PBS.
In terms of the Insulin aspart (Fiasp Penfill®) injections (human analogue), cartridges 100 units per mL, 3mL, the PBAC recommended the listing of these cartridges in November 2018 but these have not been listed on the PBS as the applicant, Novo Nordisk Pharmaceuticals Pty Ltd, did not accept the PBAC recommendations. Positive PBAC recommendations that have not been accepted by the applicant after two years are reviewed by the PBAC to determine if the recommendation should be retained. Novo Nordisk Pharmaceuticals Pty Ltd has been invited to provide a response to the PBAC on this matter and the response will be considered at the July 2023 PBAC meeting. Further information on the process for reviewing positive PBS-listing recommendations that have not been accepted by applicants is available on the PBS website at: http://www.pbs.gov.au/info/news/2021/02/amendments-to-the-process-for-reviewing-and-remaking-positiv.
I hope this information is of assistance.
Thanks David, i hope what you are saying about about number of forms a medication is provided was the limitation that stopped Novo from providing the Penfill Fiasp insulin to Australia.
I did look at the PBAC meeting agenda and it appears Eli Lilly has a new listing for a Type 2 diabetes drug Terzepatide/Mounjaro which it is providing in 6 form factors. Also just above Juno Pharm is providing Exarane in 7 form factors.
Maybe PBS has removed the >5 types compulsory discount?