We have already seen reports in Australian peer-support communities of dramatic pump failures when Apidra insulin is used in the Tandem t:slim X2!
A few people who have previously been successfully using Apidra in their pumps have been upgraded to the Tandem t:slim X2 (either through Vibe upgrades or through new purchases) and then found that the pump silently occludes within a day.
Symptoms have included hours of no insulin delivery, without occlusion alarms.
This has reportedly had severe health impacts in some cases.
But remember, the official position is that the use of Apidra in a pump is not indicated or supported within Australia. So no part of the system needs to warn you that it won’t work: you’re not “supposed” to be using Apidra in a pump anyway.
That’s despite the fact that there is a population of pump users in Australia who successfully use Apidra via “off-label” prescription by their endocrinologist. For some it’s the only insulin they can use due to allergy issues.
I trialled Apidra in Medtronic and Accu-Chek pumps earlier this year (to investigate its different activity profile) although I’ve gone back to using Humalog.
Back in July when it seemed apparent that the Tandem t:slim X2 pump would come to Australia, I mentioned that the use of Apidra in this pump was problematic (based on user feedback from the US). It seems that this has proven true here too.
What’s different with the Tandem?
It seems Apidra isn’t labelled for pump use due to the frequency of occlusions in infusion sets during Australian clinical trials, and is only labelled for injection use. Some people describe it has the insulin “crystallising” in the tubing, but that may be a simplification.
However many people in the US (and possibly Europe and other places) successfully use Apidra in pumps. At least pumps with traditional “piston”-style reservoirs.
In the US the FDA label for Apidra says the reservoir should be replaced every 2 days, and when I used Apidra for two months this year (including during my trip to Africa) I replaced the reservoir every 2 days (although I used infusion sets for 3 days). I did not encounter any occlusion problems. But I wasn’t using a Tandem pump.
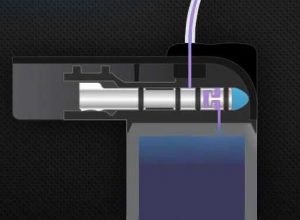
A significant difference between the t:slim X2 and other pumps is its “micro-delivery” mechanism, which shuttles tiny parcels of insulin from the bladder reservoir to the infusion set. Amongst other advantages, this allows the pump to vary infusion rates in increments as small as 0.001 U/hr (yes, that’s a thousandth of a unit). This pump mechanism is contained in the end of the reservoir.
My own hypothesis is that the tiny chamber inside the mechanism might be where Apidra is “crystallising” or otherwise causing a blockage, and this might be interfering with the pump’s occlusion-detection (given that occlusion typically occurs at the cannula or in the tubing, not in the pump itself). However I am not a Tandem engineer with complete understanding of the issues involved, so it’s just a hypothesis.
Conclusion
I WOULD NOT ATTEMPT TO USE APIDRA IN A t:slim PUMP!
If I was not able to move from Apidra to either Humalog or NovoRapid, I would not purchase a t:slim X2.
Unfortunately, there doesn’t seem to be a way around this at the moment.
AMSL (the Australian Tandem distributor) is apparently aware of the issue now, but I’ll be surprised if they have any solutions other than “don’t use Apidra”.


It doesnt crystallise, based on feedback from someone using it. It becomes a thick gel
Trying to put a gel down a short tube say in a syringe/pentip isn’t too hard
Pushing a gel down a long tube gets more and more difficult
Unlike proper liquids, gels sometimes have an ability to compress – so especially with smaller doses, with the Tandem doing lots of little bits, some might actually decompress between the little ones. Thus the occlusion may not ever be picked up.
There’s possibly a threshold when the dose goes over a certain amount that the pump then goes nup, can’t push anymore and triggers the occlusion alarm
The two cases I’ve seen (dose wise), one was easily double the other basal rate wise – the smaller never triggered, the larger did (eventually)
what about Apidra on Medtronic 670G
Apidra has definitely been used successfully by people with the 640G.
I’m sure someone’s using it with the 670G. But I don’t know specific examples.
I have been using Apidra for about 9 months in my Tandem tslim x2 without any issue. Allergic to Novolog, and homologue I was grateful to find an insulin I can take. All this negative press is anecdotal and keeps thousands of kids from using a med that can be given after they eat so you can dose accurately. You have to change the cartridge every 2 days but I am allergic to the adhesive on the cannula so need to change every 48 hrs anyway. Never have I had a single problem.
Very strange. We do of course have a few other pumps to choose from if we’re using Apidra (the “Does insulin X work in pump Y?” article went into this with the 2021 range).
Your comment was intriguing, so as a quick ad-hoc test I filled a t:slim X2 with Apidra and set it going. It occluded in the first day (although no, not everything immediately turned to gel as has often been seen). And then the next reservoir occluded multiple times. I hadn’t seen occlusion alarms for a very long time before this.
I’m glad it’s working for you at least!