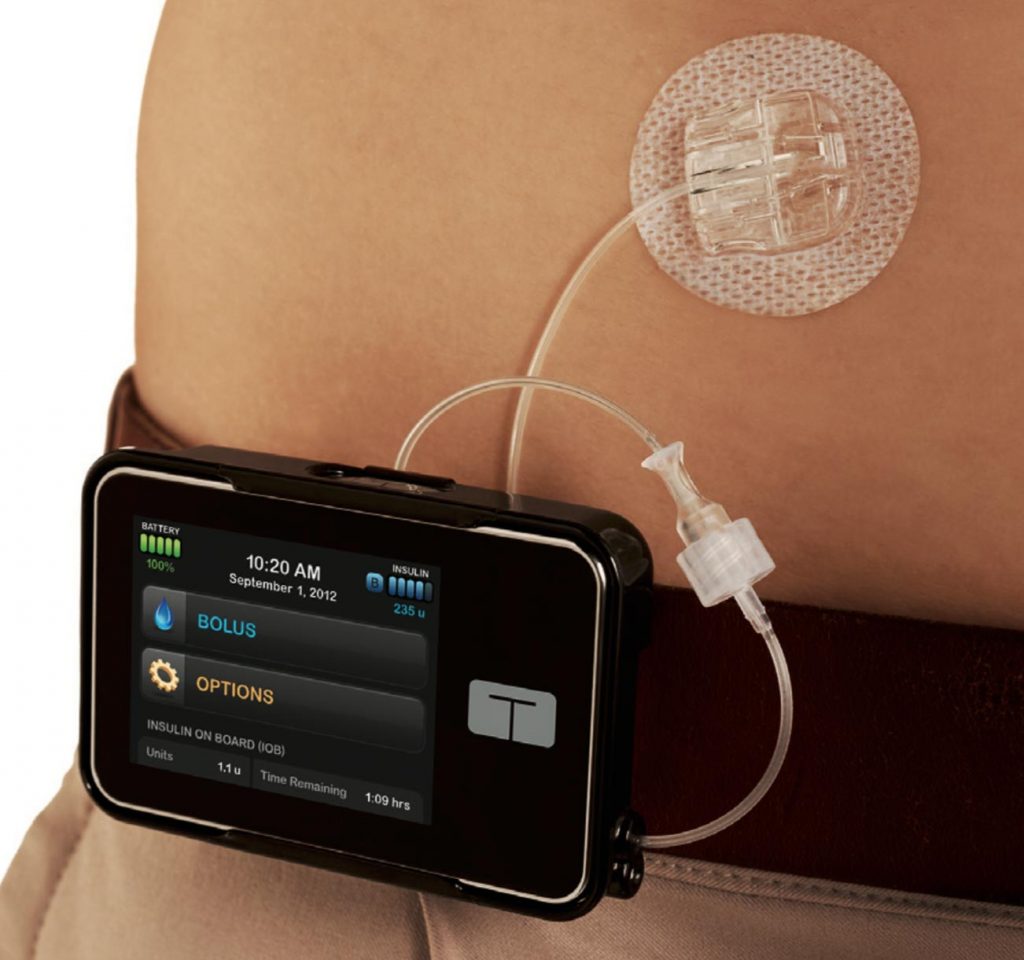
Accu-Chek Performa BG meter – a last-minute purchase
This is part travel story, and part commentary about a BG meter. Take spares For my recent African trip I assembled spares of everything, so I’d be fine if anything misbehaved. I had a backup pump, a backup OpenAPS rig, a backup CGM-collection phone, a spare G5 transmitter, spare sensors, BG meters, insulin (both sets …
Accu-Chek Performa BG meter – a last-minute purchase Read More »