NOTE: This article has been superceded by the July 2020 update.
There have of course been more changes in the Australian pump landscape since my April update. So here’s a new run-down.
There are currently 6 pumps that can be purchased new through private health insurance:
There are also some older pumps still in use, such as:
The consumables for the above pumps are available through NDSS. However, the Animas reservoirs will only be available until February 2020.
As usual I’ll run through them all with brief descriptions. Then I’ll mention other pumps we’re aware of which are not available.
For new pumps to be available in Australia, the first hurdle they need to pass is registration with the Therapeutic Goods Administration (TGA), after which doctors and suppliers are legally allowed to talk about them. The next hurdle is generally getting listing on the Federal Department of Health’s Prostheses List so they’re eligible for supply by health insurance. The Prostheses List was updated recently (with the addition of the Solo) and the next update is due in March 2020.
A note about “closed-loop” systems, those are systems which use your CGM data to dynamically adjust your pump’s delivery of insulin. These are not the mythical complete “artificial pancreas” replacement, but they do add a lot of automation to help you manage your diabetes. For information about the open protocol closed-loop systems, see my separate page on the Australian options.
Pumps available new in Australia
The following pumps are available new. Usually they’re bought through private health insurance, where they’re classified as prosthetic devices. With Australia’s updated health insurance tiers, they’re covered under Gold-level policies, plus many insurers are also covering them at lower levels (some still cover them under Bronze-with-extras). Check with your own insurer as to what their particular policies cover.
Unfortunately this is still a developing arena and insurers have up until April 2020 to restructure their policies to fit the new system.
Medtronic 670G
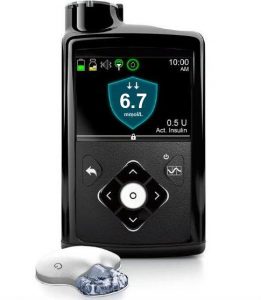 The 670G pump is Medtronic’s first closed-loop system. The pump acts as the receiver for the Guardian3 Link CGM, which it needs for the closed-loop functionality.
The 670G pump is Medtronic’s first closed-loop system. The pump acts as the receiver for the Guardian3 Link CGM, which it needs for the closed-loop functionality.
The pump is supplied with a linked “Contour Next Link 2.4” BG meter. The meter, pump, and CGM sensors communicate via a radio protocol which is not Bluetooth.
The reservoir in the 670G can hold up to 300U of insulin (it can also use a 180U reservoir), and the basal rates can be set to 0.025 U/hr increments in 30-minute blocks.
The Guardian3 CGM has sensors that are supposed to last for 7 days, and still require calibration within every 12 hours.
The 670G is not compatible with any external closed-loop systems.
With its CGM the 670G is able to have the same SmartGuard function as the 640G, as well as having “auto mode” which is their closed-loop system. Note that it is a first-generation looping system and lacks many of the configuration options and features of the open protocol loop systems, although it is approved by the regulators and available without you having to assemble it yourself.
As with any product there have been pros and cons. For example there have turned out to be various “gotchas” in use, such as when the system decides to drop out of auto mode for one of a zillion reasons you need to manually re-enable SmartGuard and not assume it’s still working.
Like all loop systems it needs “care and feeding”: hopefully people don’t assume it’s going to be a “magic pill” which will fix everything for them automatically.
Medtronic’s research into refined closed-loop functionality (for example as referenced here) will presumably make their way into future Medtronic pumps. The 670G is a fairly fixed entity.
Accu-Chek Combo
 This pump is a relatively old design, but it has proven itself as a reliable performer over the years. The Combo system is comprised of a “Spirit Combo” pump along with a “Performa Combo” BG meter which is used as a remote control so the pump doesn’t have to be brought out of your pocket in normal use.
This pump is a relatively old design, but it has proven itself as a reliable performer over the years. The Combo system is comprised of a “Spirit Combo” pump along with a “Performa Combo” BG meter which is used as a remote control so the pump doesn’t have to be brought out of your pocket in normal use.
The Spirit Combo can hold up to 315U of insulin, and the basal rates can be set to 0.01 U/hr increments in 1-hour blocks. The pump has a luer-lock connection for its infusion sets, so it can be used with any of the Roche/Accu-Chek sets along with the Unomedical (“Animas”) infusion sets, the Cleo 90 and Medtronic’s Quick-set.
Currently this is the pump I’m personally using, as an in-warranty pump that can be used with the AndroidAPS closed-loop system to integrate with my CGM.
Accu-Chek Solo
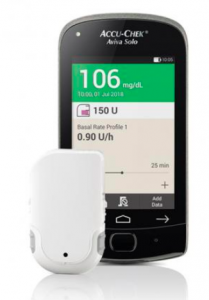 This pump has just been added to the Prostheses List at the start of November (and will thus now be funded by private health insurance). The tubeless patch pump has multiple components, starting with a cannula patch onto which the pump clips. The cannula is 90˚ teflon with two length options. There’s a disposable 200U reservoir which also includes the battery which powers the body of the pump. The pump body nominally lasts 4 months. The Aviva Solo remote controller has an integrated BG meter that uses Accu-Chek’s Aviva strips (although you can enter corrections using a BG value you’ve retrieved from another meter or CGM).
This pump has just been added to the Prostheses List at the start of November (and will thus now be funded by private health insurance). The tubeless patch pump has multiple components, starting with a cannula patch onto which the pump clips. The cannula is 90˚ teflon with two length options. There’s a disposable 200U reservoir which also includes the battery which powers the body of the pump. The pump body nominally lasts 4 months. The Aviva Solo remote controller has an integrated BG meter that uses Accu-Chek’s Aviva strips (although you can enter corrections using a BG value you’ve retrieved from another meter or CGM).
 The Solo pump is not waterproof: you are expected to remove it (leaving the cannula patch in place) for swimming and showers. This is primarily because the battery has to use a “zinc-air” chemistry to get enough capacity, and without access to air the battery stops working.There’s a small vent on the outside of the pump, that if you covered with tape would kill the pump.
The Solo pump is not waterproof: you are expected to remove it (leaving the cannula patch in place) for swimming and showers. This is primarily because the battery has to use a “zinc-air” chemistry to get enough capacity, and without access to air the battery stops working.There’s a small vent on the outside of the pump, that if you covered with tape would kill the pump.
Because the pump body lasts for 4 months, when you buy the pump it looks like each year you will receive three bodies. Plus if it’s the first time you’ve bought the pump you’ll receive a handset.
Earlier this year I wrote about my experience wearing a dummy Solo.
Tandem t:slim X2
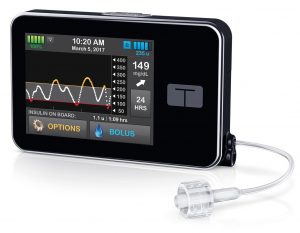 This pump is a small pump with a touchscreen interface. The aluminium case is very robust, and gives it a slight heft in your hand. The internal battery is recharged by USB (typically topped up at least every few days).
This pump is a small pump with a touchscreen interface. The aluminium case is very robust, and gives it a slight heft in your hand. The internal battery is recharged by USB (typically topped up at least every few days).
The insulin reservoir can hold up to 300U. The basal rate can be set with a tiny increment of 0.001 U/hr in 16 arbitrary time blocks. However, the minimum basal rate is 0.100 U/hr (or 0), and this can cause issues for some paediatric use.
Note that even if your basal rate is 0.6 U/hr, that minimum 0.1 still applies to any temp basal. Thus the lowest TBR you’d be able to apply would be 17%. Not 10%. If your basal rate was 0.3 U/hr you’d only be able to drop to 34%.
The Tandem reservoirs are compatible only with the Tandem t:lock infusion sets. Some people are still using the previous reservoirs that could be used with any luer-lock infusion set, but those have been discontinued.
Note that Apidra insulin is not compatible with the Tandem reservoirs (it’s not officially listed for pump use in Australia, but is sometimes used in other pumps). Apidra fails catastrophically in this pump. Fiasp is officially not supported either, although some people apparently do use it successfully.
A touted feature of this pump is the ability to update its firmware at home via USB without having to have the whole pump replaced/serviced, and this will be used to add features to the pump during its 4-year warranty period. Right now the pump can act as a Dexcom G5 CGM receiver. When the Dexcom G6 is introduced to Australia (hopefully in 2019, but the year is running out) the firmware will be updated to support G6 along with Tandem’s “Basal-IQ” low-glucose-suspend function (once that update also receives TGA approval). This suspends and resumes basal insulin as required to keep your BG above 4.4 mmol/l. It was released to US users in August 2018, and has received a lot of positive feedback.
 Tandem promising the subsequent “Control-IQ” firmware update, which is their closed-loop pump control system, working to keep your BG in range all the time. Many people are understandably excited about this. This has been submitted to the US regulator (FDA) for approval, but has not yet reached any market. Tandem have indicated it should be late 2019 in the US, but we’ll have to wait to see how good that estimate is. AMSL have stated that both the Basal-IQ and Control-IQ updates will be free in Australia (although users will presumably need to go through additional training and have medical signoff to receive the update). As long as your pump is still in warranty.
Tandem promising the subsequent “Control-IQ” firmware update, which is their closed-loop pump control system, working to keep your BG in range all the time. Many people are understandably excited about this. This has been submitted to the US regulator (FDA) for approval, but has not yet reached any market. Tandem have indicated it should be late 2019 in the US, but we’ll have to wait to see how good that estimate is. AMSL have stated that both the Basal-IQ and Control-IQ updates will be free in Australia (although users will presumably need to go through additional training and have medical signoff to receive the update). As long as your pump is still in warranty.
The t:slim X2 is rated as IP67 waterproof, which is not quite as robust as the IPX8 rating of most other current pumps. See this article for a discussion of waterproof ratings. Mind you, the IP67 rating does still allow for short-term immersion in water up to a metre deep.
The t:slim pump is not compatible with any open protocol closed-loop system.
YpsoPump
 The YpsoPump (from Ypsomed) is a tiny unit which links to the MyLife app on your phone via Bluetooth. Currently the app has a one-way connection: it needs the user to use the pump’s touchscreen to issue boluses, but does most of the the rest of the management and connection from the phone (including carb/bolus calculations). An update to the pump has been promised which will give the app full remote control (and probably integrate with a CGM) but we do not yet know a date for this.
The YpsoPump (from Ypsomed) is a tiny unit which links to the MyLife app on your phone via Bluetooth. Currently the app has a one-way connection: it needs the user to use the pump’s touchscreen to issue boluses, but does most of the the rest of the management and connection from the phone (including carb/bolus calculations). An update to the pump has been promised which will give the app full remote control (and probably integrate with a CGM) but we do not yet know a date for this.
The reservoir holds up to 160U of insulin, and the basal rates can be set to 0.01 U/hr increments in 1-hour blocks. The pump uses the Orbit infusion sets (with steel and teflon options) although more options may be introduced at some point.
The reservoir is smaller than in some other pumps, although 160U is enough for many people’s requirements. Plus the reservoirs are glass (unlike the plastic of other pumps) and as such are approved for longer-term insulin storage. The tested and approved use includes the ability to pre-fill reservoirs and keep them in your fridge for up to 4 weeks.
The YpsoPump cannot currently be used with any open protocol closed-loop systems, but Ypsomed have committed to working with the JDRF Open Protocol Initiative. Development work is ongoing.
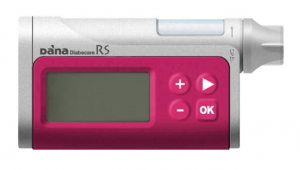
DANA RS
The DANA RS is in an unusual position because the supplier (Managing Diabetes) is not currently pursuing sales, although the RS is TGA-approved and like the other pumps here it’s on the list of pumps that private health insurance will purchase.
It’s primarily being sold to people who are seeking it out because of its official support for the AndroidAPS open protocol loop system.
The DANA RS has a 300U insulin reservoir, and basal rates can be set to 0.01 U/hr increments in 1-hour blocks. It uses DANA-specific infusion sets (including a teflon one made by Ypsomed and equivalent to the OrbitSoft set). The OrbitMicro steel cannulae (available on NDSS without tubing) can be used with the DANA tubing if you need to use those. The special batteries for the pump are supplied in each box of reservoirs.
Other pumps in use in Australia
While not available new, the following pumps are still in use by many people.
Medtronic 640G
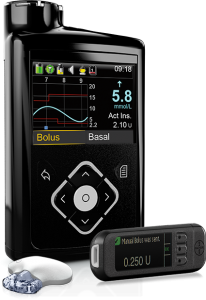 This pump originally integrated with Medtronic’s Guardian2 Link CGM, but also supports the Guardian3 Link that was introduced with the 670G.
This pump originally integrated with Medtronic’s Guardian2 Link CGM, but also supports the Guardian3 Link that was introduced with the 670G.
It uses that data for “SmartGuard” which is their predictive-low-glucose-suspend (sometimes referred to as a “hypo minimiser”) although this function is dependent on keeping the CGM calibrated and accurate.
The pump is supplied with the same BG meter as the 670G, and the reservoir capacity and consumables are the same.
The 640G is not compatible with any closed-loop system.
Medtronic Paradigm “Veo” (554 or 754 models)
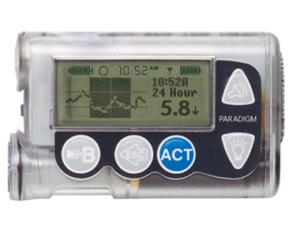 These were Medtronic’s previous generation of pumps.
These were Medtronic’s previous generation of pumps.
The reservoir in the 554 can hold up to 180U of insulin, and the 754 can hold up to 300U (or use the 180U option). The basal rates can be set to 0.025 U/hr increments in 30-minute blocks.
The pumps support Medtronic’s old “Enlite” CGM system (uses the same sensors as the Guardian2 CGM, but a different transmitter) and they have a primitive low-glucose-suspend function (which suspends when you get to the boundary point, not when the pump predicts you’re soon going to get there).
These pumps were originally rated with IP67 dust/waterproofing, but especially given their age I would not regard any of them as waterproof today.
The latest firmware versions of these pumps are not compatible with any open protocol closed-loop systems. Older versions with firmware up to 2.7A are “loopable” and supported by both the Loop and OpenAPS closed-loop systems. As are many other older Paradigm models.
Animas Vibe
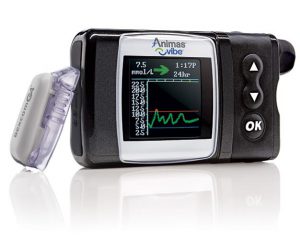 Animas shut down and discontinued this pump (back in early 2018). The transition has taken a while, but by now the Vibe’s time is now almost up. The Vibe reservoirs are still available through NDSS, but only until February 2020!
Animas shut down and discontinued this pump (back in early 2018). The transition has taken a while, but by now the Vibe’s time is now almost up. The Vibe reservoirs are still available through NDSS, but only until February 2020!
By now all in-warranty users should have been transitioned by AMSL to Tandem t:slim X2 pumps for the remainder of their warranty period. There are of course some people who have not been able to transition (e.g. factors such as the minimum basal rate of the t:slim can be an issue).
The Vibe reservoir can hold up to 200U, and the basal rates can be set to 0.025 U/hr increments in 30-minute blocks. The pump has an integrated receiver for the Dexcom G4 CGM system. The Vibe is NOT compatible with any closed-loop systems.
DANA R
This pump looks almost identical to the RS. The main differences are the use of Bluetooth 2 rather than the lower-power Bluetooth LE, and a correspondingly-worse battery life (approximately a third of the lifespan).
The DANA R is supported by the AndroidAPS closed-loop system.
A non-T1D pump
V-Go
 The V-Go is available in Australia, but is designed for use in Type 2 diabetes, not Type 1. It is a simply mechanical (spring-driven) device for 1-per-day use, with a fixed infusion rate. I have previously written about the V-Go.
The V-Go is available in Australia, but is designed for use in Type 2 diabetes, not Type 1. It is a simply mechanical (spring-driven) device for 1-per-day use, with a fixed infusion rate. I have previously written about the V-Go.
Other insulin pumps
That’s it for the current Australian pump options, but there are some other pumps around the world which Australian folk keep asking about. The following pumps are not available in Australia, and there are no known plans for Australian access yet.
So a quick overview:
Insulet OmniPod
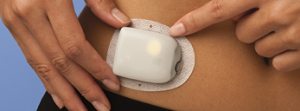 This tubeless patch pump is available in the US and Europe, but although medically it’s been approved for use in Australia since 2012 it has never been sold here. The economic model simply doesn’t fit the funding model used in Australia.
This tubeless patch pump is available in the US and Europe, but although medically it’s been approved for use in Australia since 2012 it has never been sold here. The economic model simply doesn’t fit the funding model used in Australia.
Every 3 days a whole new pump is required: the only permanent piece is the “PDM” remote controller. This would shift most of the cost from your health insurance to the NDSS which supplies consumables, and no-one’s been able to work out a compromise. It also produces a lot of hard-to-recycle waste. But the pump can be worn during swimming/showering/etc. If the pump detaches, it can’t be re-attached.
Incidentally, the old version’s internal name is “Eros”, and uses 433 MHz radio to link the PDM and the pumps. Their new “DASH” system instead uses Bluetooth but it otherwise the same. The Eros OmniPods can be used with opensource closed-loop systems (primarily Loop at the moment). The DASH will be used by Tidepool’s future commercial closed-loop version of Loop, as well as Insulet’s own Horizon closed-loop.
Accu-Chek Insight
 This pump is used in Europe, but not available here (although it was registered with the TGA back in 2015, as well as added to the Prostheses List). It uses essentially the same cartridges as the YpsoPump, but the pre-filled Novorapid “PumpCart”s used for it in Europe are not available in Australia. The end of the pump is actually part of the infusion set tubing (the pump needs its own collection of infusion sets). The Insight is supported by the AndroidAPS closed-loop software.
This pump is used in Europe, but not available here (although it was registered with the TGA back in 2015, as well as added to the Prostheses List). It uses essentially the same cartridges as the YpsoPump, but the pre-filled Novorapid “PumpCart”s used for it in Europe are not available in Australia. The end of the pump is actually part of the infusion set tubing (the pump needs its own collection of infusion sets). The Insight is supported by the AndroidAPS closed-loop software.
I do not expect to see the Insight appear in Australia.
EOFlow patch pump
 This patch pump from Korea got some press in March 2019 when it got “Breakthrough Device” designation from the US FDA. However it is still a long way from market. Development plans apparently include integration with the POCtech CGM into a single unit.
This patch pump from Korea got some press in March 2019 when it got “Breakthrough Device” designation from the US FDA. However it is still a long way from market. Development plans apparently include integration with the POCtech CGM into a single unit.
Medtrum A6
 This tubeless patch pump from China has had TGA registration for some time, but does not seem to be on anyone’s marketing plans. Reports from overseas users characterise it as a relatively primitive device (especially its controller device).
This tubeless patch pump from China has had TGA registration for some time, but does not seem to be on anyone’s marketing plans. Reports from overseas users characterise it as a relatively primitive device (especially its controller device).
Currently I do not expect to see the Medtrum reach the Australian market.
Kaleido
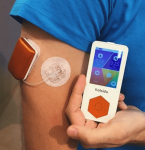 Another European patch pump, Kaleido has a similar form factor to the Cellnovo system, but also allows you to use longer tubing for flexibility in how you wear it. It’s the pump Diabeloop in France chose for their DBLG1 closed-loop system (which did use Cellnovo during development).
Another European patch pump, Kaleido has a similar form factor to the Cellnovo system, but also allows you to use longer tubing for flexibility in how you wear it. It’s the pump Diabeloop in France chose for their DBLG1 closed-loop system (which did use Cellnovo during development).
Currently I do not expect to see Kaleido reach Australia.
DANA-i
 This will be the next pump from SOOIL. It has been shown at some trade shows so far, but has not reached markets yet. It’s being targetted at regulatory approval in the US and Europe. It can be seen as the next generation of the RS, but I believe it uses a standard AAA battery and may have more options for infusion sets.
This will be the next pump from SOOIL. It has been shown at some trade shows so far, but has not reached markets yet. It’s being targetted at regulatory approval in the US and Europe. It can be seen as the next generation of the RS, but I believe it uses a standard AAA battery and may have more options for infusion sets.
The DANA-i is another of the pumps that will be aiming at use with commercial closed-loop systems. I gather that unlike the earlier R and RS pumps the manufacturer will not be supporting its use in opensource software such as AndroidAPS.
Medtronic 780G
This is Medtronic’s touted next closed-loop pump. It (or parts of it) are being trialled at some locations overseas. It apparently has Bluetooth for connecting to your phone, and a more-advanced closed-loop algorithm than the 670G. But it looks almost identical to the 670G (and 640G).
When it eventually gets closer to the US market I’ll be able to describe more about it.
That’s it for now
Hopefully our pump options will continue to evolve over time, so expect more updates next year!


Anyone have any idea when the DANA people will start selling again. I want one and can get a new pump in January 2020.
The DANA RS has been being sold for a while now. Contact Managing Diabetes if you’re interested in it.
I just got to handle one of the accu-chek solo pumps – the patch pump looks and feels great though the hand piece seems unnecessarily bulky. The rep told me it is $2500 the first year inc hand piece – which has 4 years warranty, $2000 for each of the 3 years after that, so it is similarly prices to other pumps (except you cant cancel your medical insurance!)
Question is… is there any patch you know of, so your mobile phone can be used as the hand piece with 5G cgm?
Thank you for these great articles David.
No, the Solo is the only patch pump available in Australia at the moment. And it doesn’t link to your phone.
As always a great article. Thanks David!
Thanks David. This has been so very useful.
I am about to start my pump journey with YpsoPump having persisted with MDI (pen) therapy for over 26 years now. I use Libre FGM and will follow Ypsomed’s and others (reluctantly perhaps) embrace an open ecosystem
David – you wrote that the new Dana will not support AAPS. Where have you got this information from please. I was hoping that this new pump addresses the known issues of the current model, but still giving us the AAPS option. Without it, I wouldn’t make the switch.
In search of commercial approvals and partnerships with approved loop systems (which is a much bigger market than the opensource community) the company is unlikely to support AAPS in the way they have so far. This is information from people directly in a position to know.
Of course, that’s not to say that someone won’t work out how to talk to it and will add support for it to AAPS. That’s theoretically possible (depending on what protocols it uses: the current DANA RS protocol is quite old and actually comes from a time before Bluetooth LE). But the company won’t be supporting/supplying code like they have in the past.
Thanks David, very helpful, saved me a lot of research !!
Hi David, I thought a while back there was a pump where u could use the glass 300U vial directly in pump meaning no more filling cartridges, and I incorrect?
A looong time ago there was the Disetronic D-Tron Plus? Nothing since then that I’m aware of.
do you know if the accu check solo is available to buy in Australia?
Indeed. Since November last year. I know a few people using them.
I’m not sure if they’re currently rolling more out or whether they’re in a supply lull (pumps like that sometimes come into the country in batches). Contact your local Accu-Chek rep to find out.
I’m a long time Minimed pump user, but I have been interested in changing since I find Medtronic’s marketing operation toxic. I tested out the main competitor: Tandem t:slim; but it didn’t meet my expectations. I am now considering the Ypsomed pump, but I wondered if you or anyone else knows what the market shares are for each manufacturer in Australia looks like? Even if I like the Ypsomed, if there are only 150 customers in Australia, I am a bit nervous about jumping on board. It would be all too easy for Ypsomed to pull out of the market if they don’t see it shaping up the way they expected after the launch a couple of years ago.
No I don’t know the numbers on that. Companies tend to not publish that sort of info.
But personally I’m not too concerned about Ypsomed disappearing. I’ve seen their operation and spoken with the big bosses overseas. As far as I can tell they’re here to stay.